chlorpyrifos oxon
Synonyms: "o,o-diethyl o-3,5,6-trichloro-2-pyridyl phosphat", "diethyl 3,5,6-trichloro-2-pyridyl phosphate", "dursbanoxon", "fospirate-ethyl", "dursban oxygen analog", "chloropyrifos oxygen analog"
Source: chlorpyrifos oxon is a metabolite of chlorpyrifos.
Identifiers:
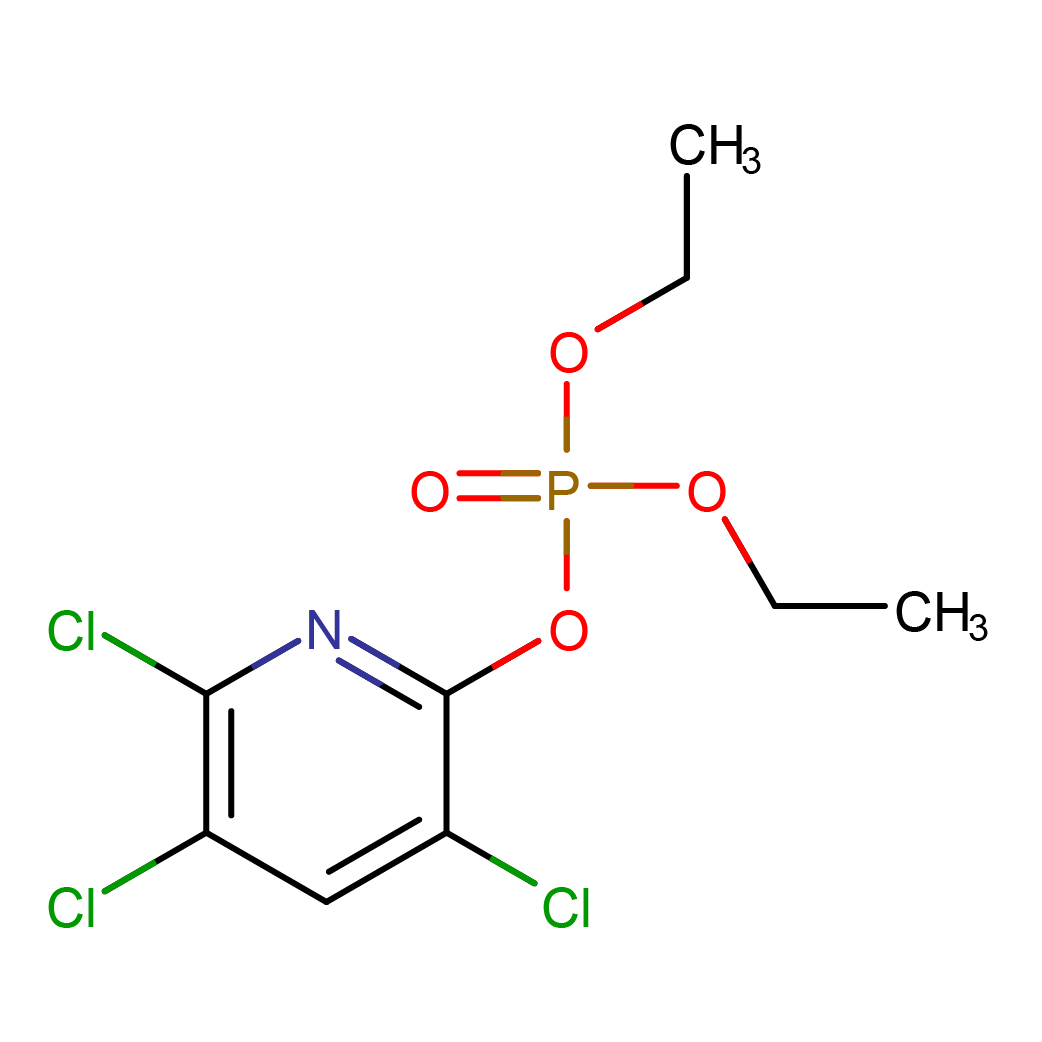
IUPAC Name: diethyl (3,5,6-trichloropyridin-2-yl) phosphate
CAS Number: 5598-15-2
PubChem ID: 21804
InChiKey: OTMOUPHCTWPNSL-UHFFFAOYSA-N
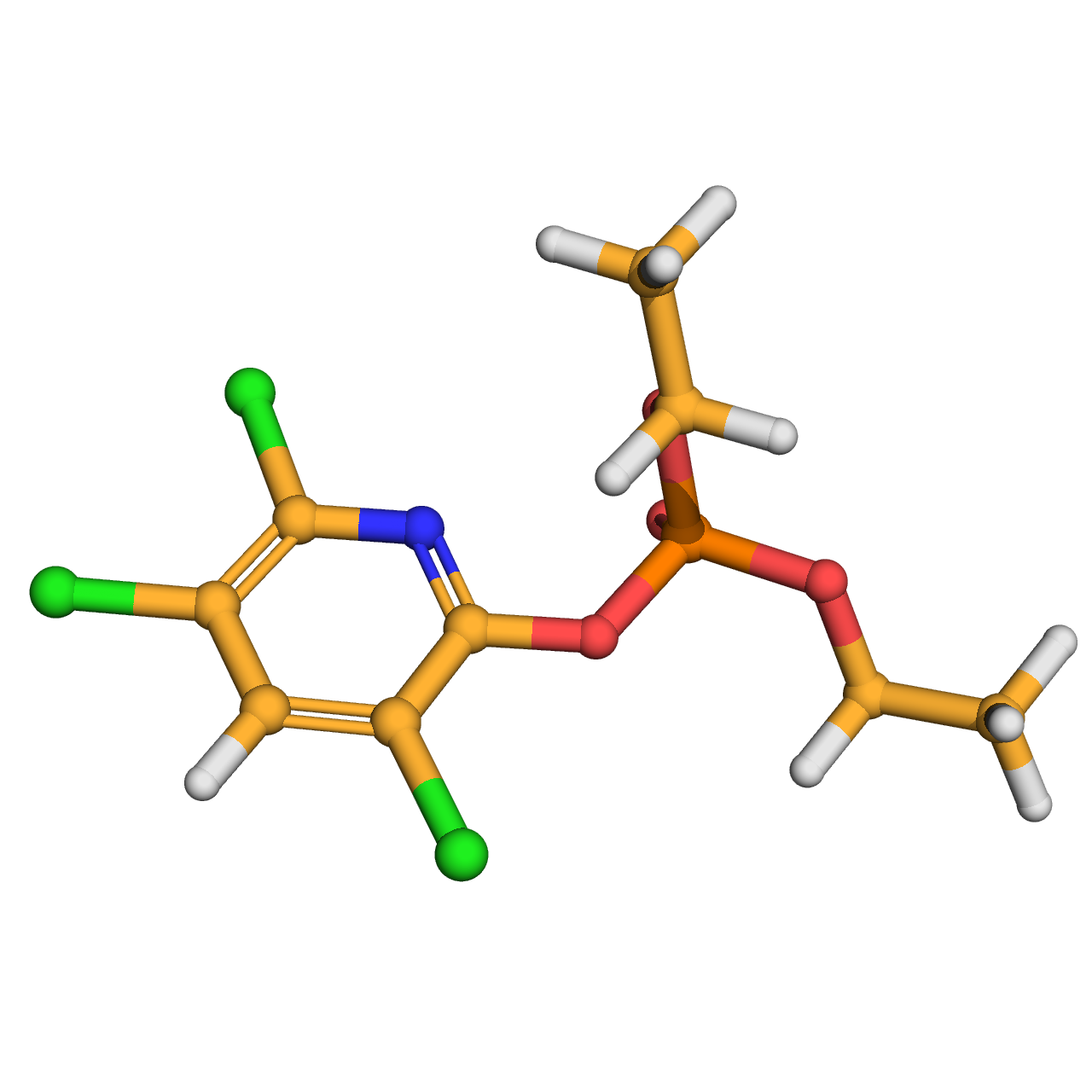
Canonical SMILES: CCOP(=O)(OCC)OC1=NC(=C(C=C1Cl)Cl)Cl
Structural Properties:
Molecular Formula: C9H11Cl3NO4P
Molecular Weight: 334.521
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. 2002. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol Lett 135(1-2):89-93, DOI: 10.1016/S0378-4274(02)00251-5.
Schuh RA, Lein PJ, Beckles RA, Jett DA. 2002. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol 182(2):176-185.
External Links

2D-structure
3D-structure