sumithrin
Synonyms: "phenothrin", "phenothrine", "phenoxythrin", "pibutin", "sumitrin", "wellcide", "duet", "anchimanaito 20S"
Source: sumithrin is a synthetic pyrethroid that kills adult fleas and ticks.
Identifiers:
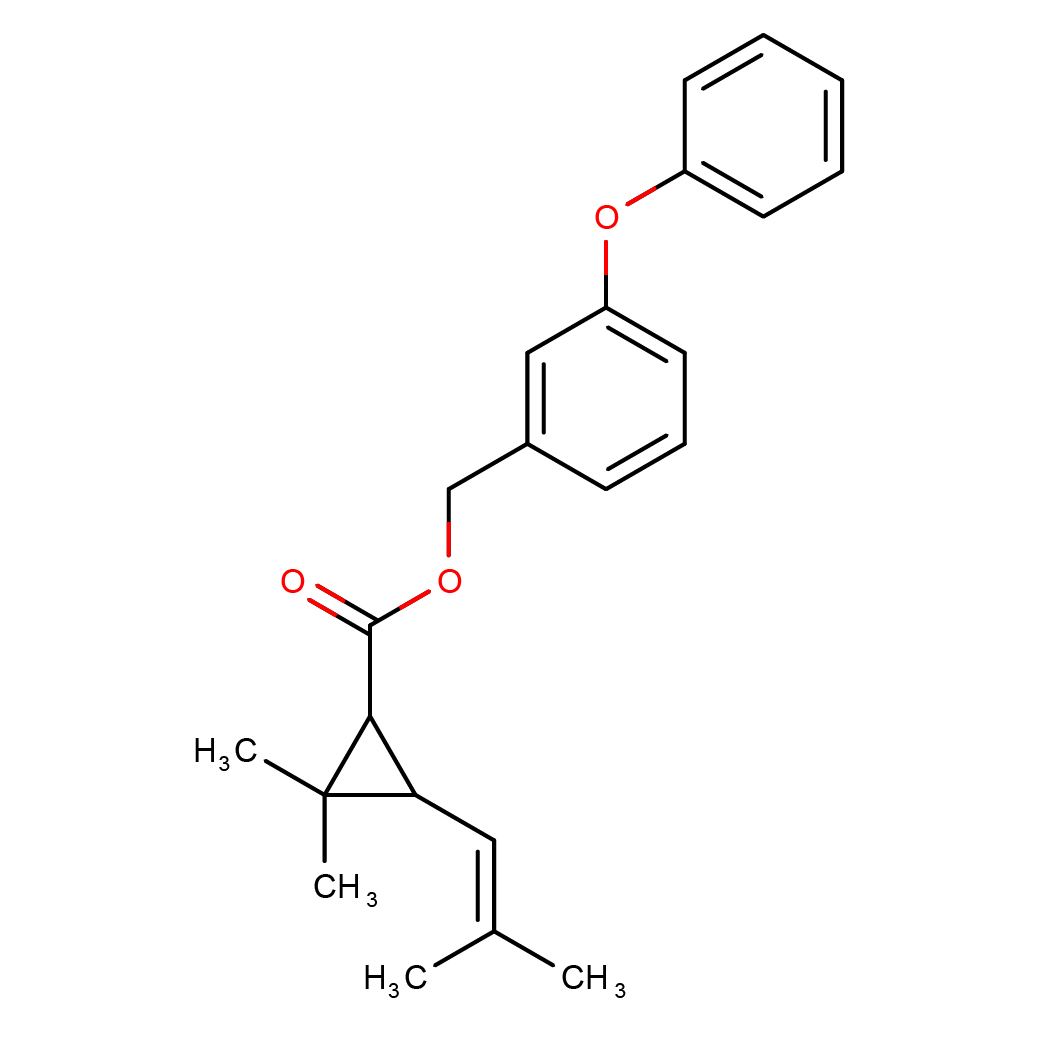
IUPAC Name: (3-phenoxyphenyl)methyl
CAS Number: 26002-80-2
PubChem ID: 4767
InChiKey: SBNFWQZLDJGRLK-UHFFFAOYSA-N
Canonical SMILES: CC(=CC1C(C1(C)C)C(=O)OCC2=CC(=CC=C2)OC3=CC=CC=C3)C
Structural Properties:
Molecular Formula: C23H26O3
Molecular Weight: 350.451
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 1
Number of atoms different from hydrogen: 26
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Eil C, Nisula BC. 1990. The binding properties of pyrethroids to human skin fibroblast androgen receptors and to sex hormone binding globulin. J Steroid Biochem 35(3/4):409-414.
Garey J, Wolff MS. 1998. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochemical & Biophysical Research Communications 251(3):855-859.
Go V, Garey J, Wolff MS, Pogo BGT. 1999. Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line. Environ Health Perspect 107(3):173-177.
External Links

2D-structure
3D-structure