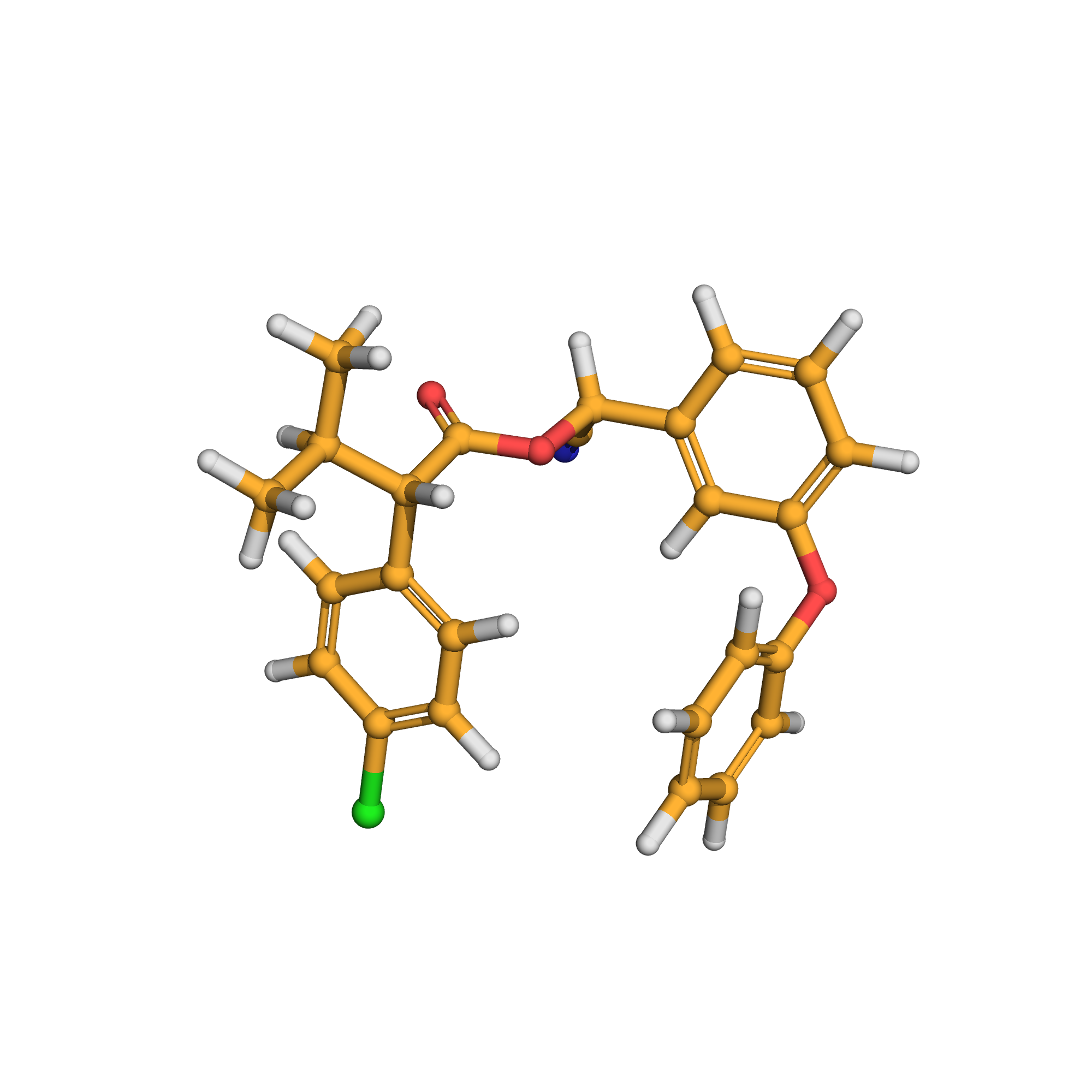
esfenvalerate
Synonyms: "fenvalerate", "asana", "(S)-cyano(3-phenoxyphenyl)methyl (2S)-2-(4-chlorophenyl)-3-methylbutanoate", "(S,S)-fenvalerate"
Source: esfenvalerate is a synthetic pyrethroid insecticide.
Identifiers:
IUPAC Name: [(S)-cyano-(3-phenoxyphenyl)methyl](2S)-2-(4-chlorophenyl)-3-methylbutanoate
CAS Number: 66230-04-4
PubChem ID: 10342051
InChiKey: NYPJDWWKZLNGGM-RPWUZVMVSA-N
Canonical SMILES: CC(C)C(C1=CC=C(C=C1)Cl)C(=O)OC(C#N)C2=CC(=CC=C2)OC3=CC=CC=C3
Structural Properties:
Molecular Formula: C25H22ClNO3
Molecular Weight: 419.900
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 30
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Barry MJ, O'Halloran K, Logan DC, Ahokas JT, Holdway DA. 1995. Sublethal effects of esfenvalerate pulse-exposure on spawning and non-spawning Australian crimson-spotted rainbowfish (Melanotaenia fluviatilis). Arch Environ Contam Toxicol 28(4):459-463.
Tanner DK, Knuth ML. 1996. Effects of esfenvalerate on the reproductive success of the bluegill sunfish, Lepomis macrochirus in littoral enclosures. Arch Environ Contam Toxicol 31(2):244-251.
External Links

2D-structure
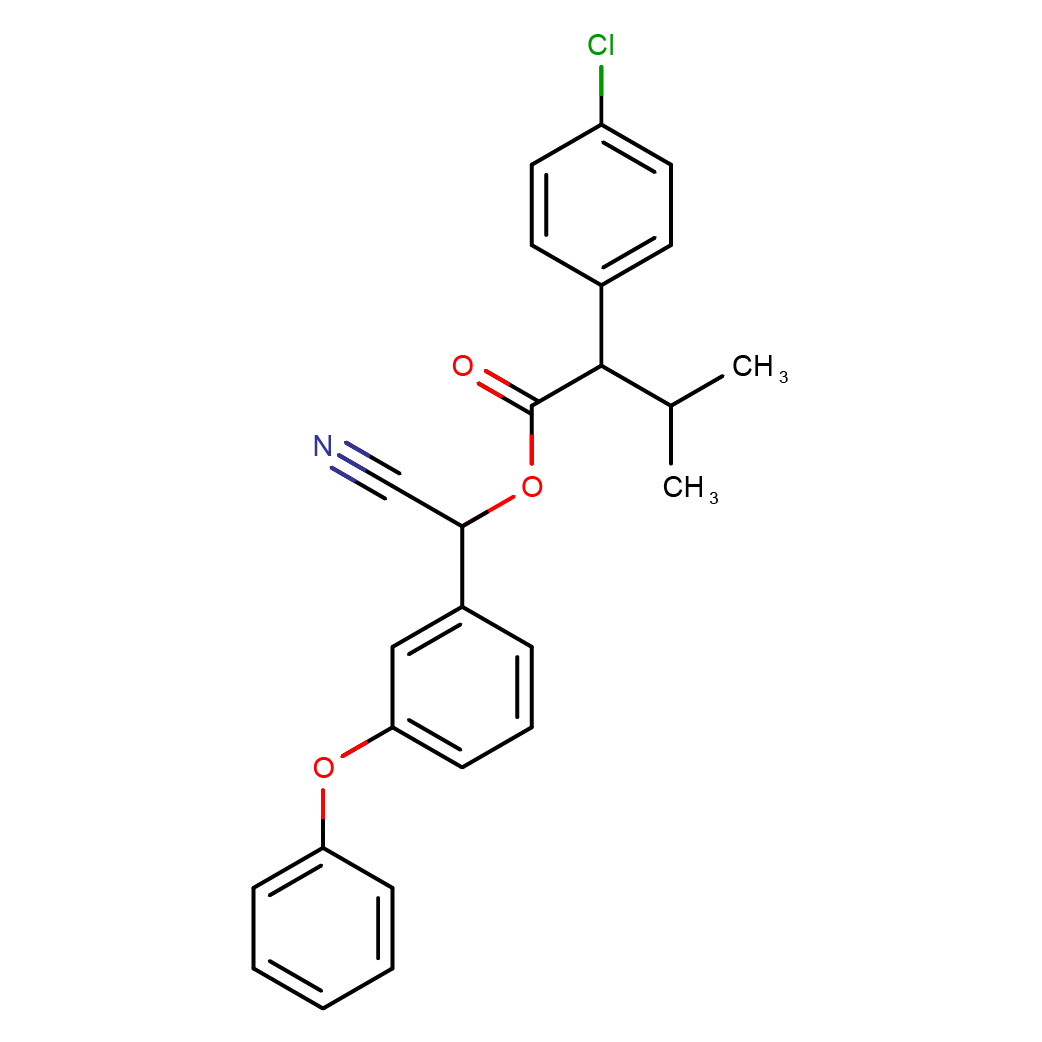
3D-structure