Aroclor 1254
Synonyms: "2,2',3,3',4-pentachlorobiphenyl", "2,2',3,3',4-pentachloro-1,1'-biphenyl"
Source: aroclor 1254 is used in pesticides as well as in various industrial applications.
Identifiers:
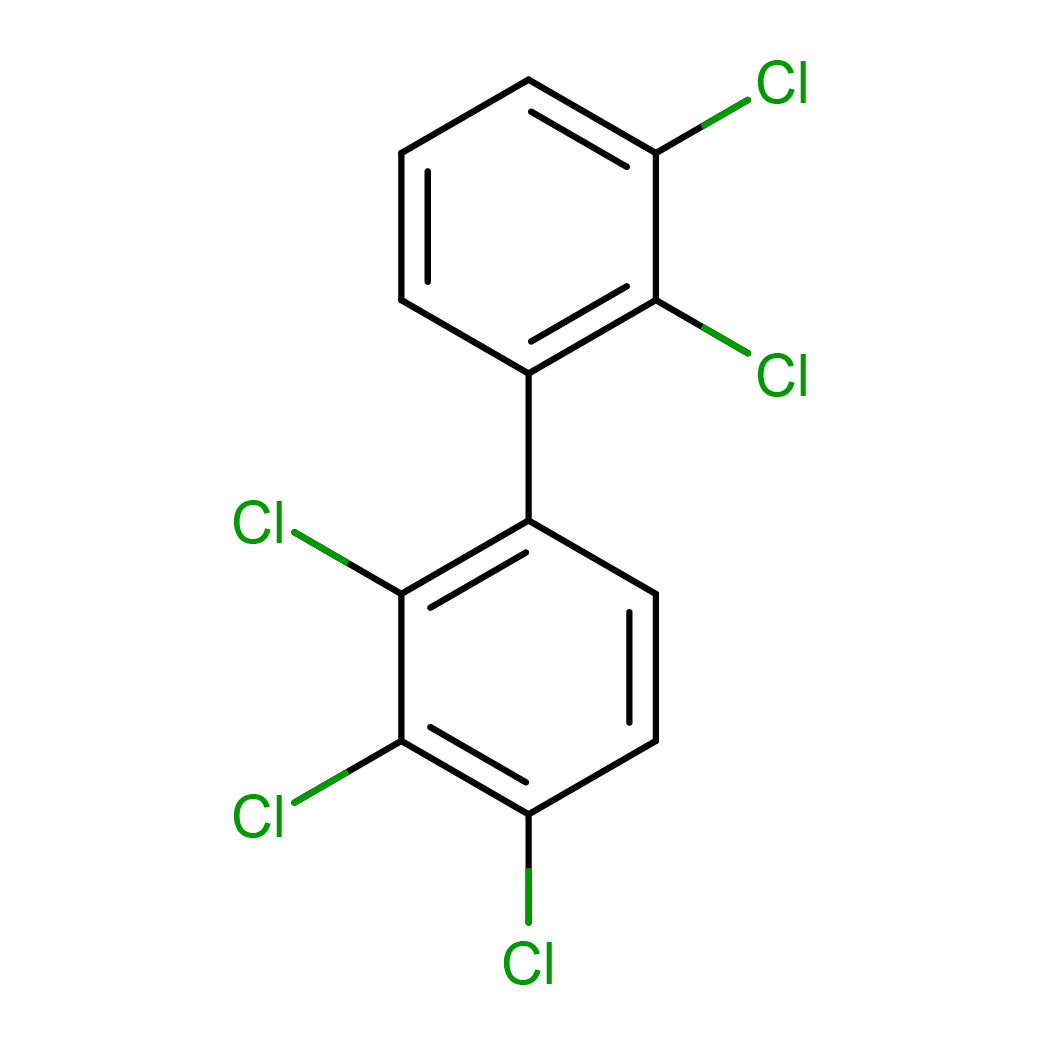
IUPAC Name: 1,2,3-trichloro-4-(2,3-dichlorophenyl)benzene
CAS Number: 11097-69-1
PubChem ID: 40470
InChiKey: AUGNBQPSMWGAJE-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=C(C(=C1)Cl)Cl)C2=C(C(=C(C=C2)Cl)Cl)Cl
Structural Properties:
Molecular Formula: C12H5Cl5
Molecular Weight: 326.433
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 17
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hornhardt S, Jenke HS, Michel G. 1994. Polychlorinated biphenyls modulate protooncogene expression in Chang liver cells. FEBS Lett 339(1-2):185-188.
Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC. 2002. Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol Appl Pharmacol 179(3):185-194.
Zoeller RT, Dowling AL, Vas AA. 2000. Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 141(1):181-189.
External Links


2D-structure
3D-structure