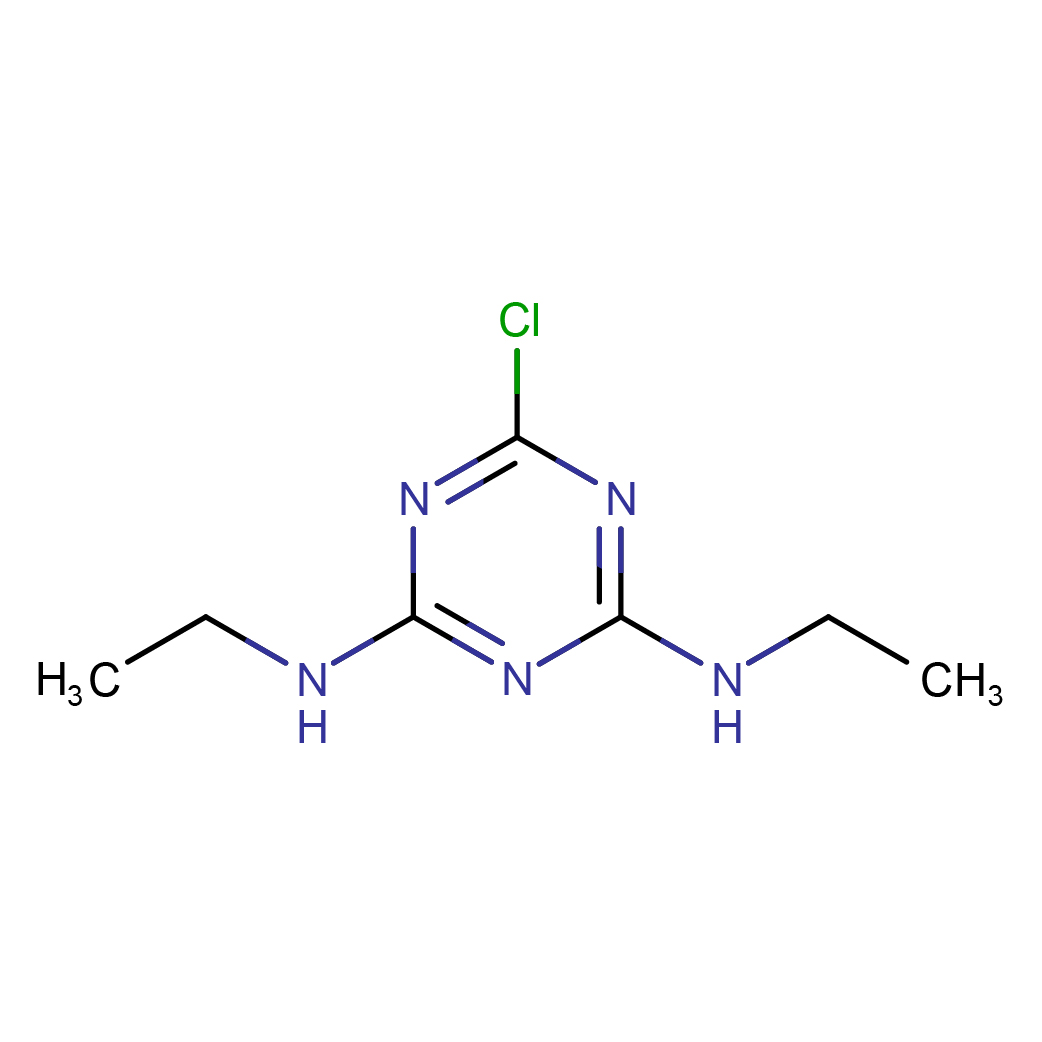
simazine
Synonyms: "gesatop", "princep", "simanex", "aquazine", "batazina", "herbazin", "symazine", "tafazine"
Source: simazine is an herbicide used to control broad-leaf weeds and annual grasses in crop fields such as fruit orchards.
Identifiers:
IUPAC Name: 6-chloro-2-N,4-N-diethyl-1,3,5-triazine-2,4-diamine
CAS Number: 122-34-9
PubChem ID: 5216
InChiKey: ODCWYMIRDDJXKW-UHFFFAOYSA-N
Canonical SMILES: CCNC1=NC(=NC(=N1)Cl)NCC
Structural Properties:
Molecular Formula: C7H12ClN5
Molecular Weight: 201.657
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 5
Number of atoms different from hydrogen: 13
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Didier R, Lutz-Ostertag Y. 1972. Action of simazine on the genital tract of embryos from chicken and quail in vivo. C R Seances Soc Biol Fil 166(12):1691-1693.
Fan WQ, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, Komatsu T, Morohashi K-I, Hayes TB, Takayanagi R, Nawata H. 2007. Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ Health Perspect 115(5):720-727.
Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den Berg M. 2001. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect 109(10):1027-1031.
Tran DQ, Kow KY, McLachlan JA, Arnold SF. 1996. The inhibition of estrogen receptor-mediated responses by chloro-s-triazine-derived compounds is dependent on estradiol concentration in yeast. Biochemical & Biophysical Research Communications 227(1):140-146.
External Links


2D-structure
3D-structure