fenoxycarb
Synonyms: "insegar", "varikill", "fenasulam", "pictyl", "pyctyl", "ethyl (2-(4-phenoxyphenoxy)ethyl)carbamate", "N-(2-(p-phenoxyphenoxy)ethyl)carbamic acid", "(2-(4-phenoxyphenoxy)ethyl)carbamic acid ethyl ester"
Source: fenoxycarb is a carbamate insect growth regulator.
Identifiers:
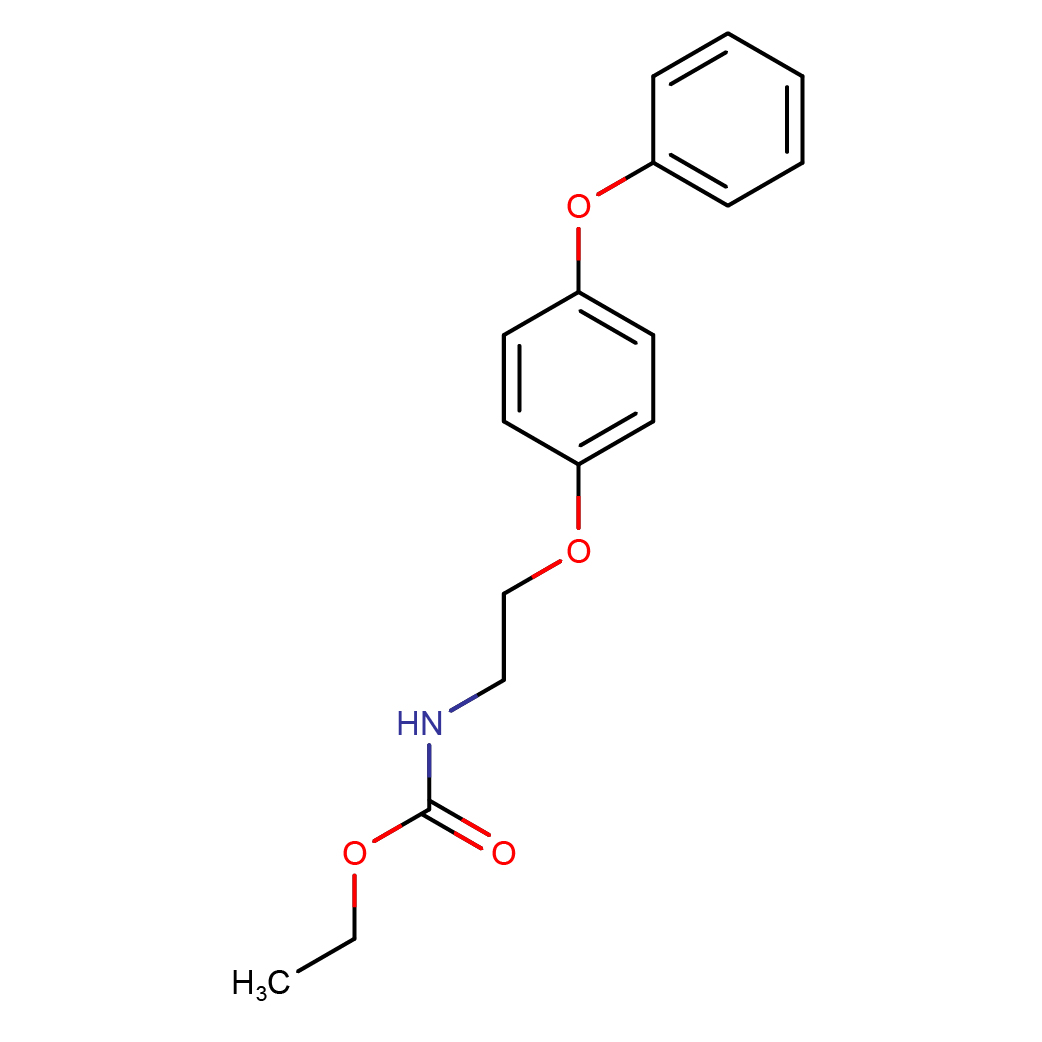
IUPAC Name: ethyl N-[2-(4-phenoxyphenoxy)ethyl]carbamate
CAS Number: 72490-01-8 (formerly 79127-80-3)
PubChem ID: 51605
InChiKey: HJUFTIJOISQSKQ-UHFFFAOYSA-N
Canonical SMILES: CCOC(=O)NCCOC1=CC=C(C=C1)OC2=CC=CC=C2
Structural Properties:
Molecular Formula: C17H19NO4
Molecular Weight: 301.337
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 2
Number of atoms different from hydrogen: 22
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Schmuck G, Mihail F. 2004. Effects of the carbamates fenoxycarb, propamocarb and propoxur on energy supply, glucose utilization and SH-groups in neurons. Arch Toxicol 78(6):330-337.
Smulders CJ, Bueters TJ, Van Kleef RG, Vijverberg HP. 2003. Selective effects of carbamate pesticides on rat neuronal nicotinic acetylcholine receptors and rat brain acetylcholinesterase. Toxicol Appl Pharmacol 193(2):139-146.
Tatarazako N, Oda S, Watanabe H, Morita M, Iguchi T. 2003. Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere 53(8):827-833.
External Links


2D-structure
3D-structure