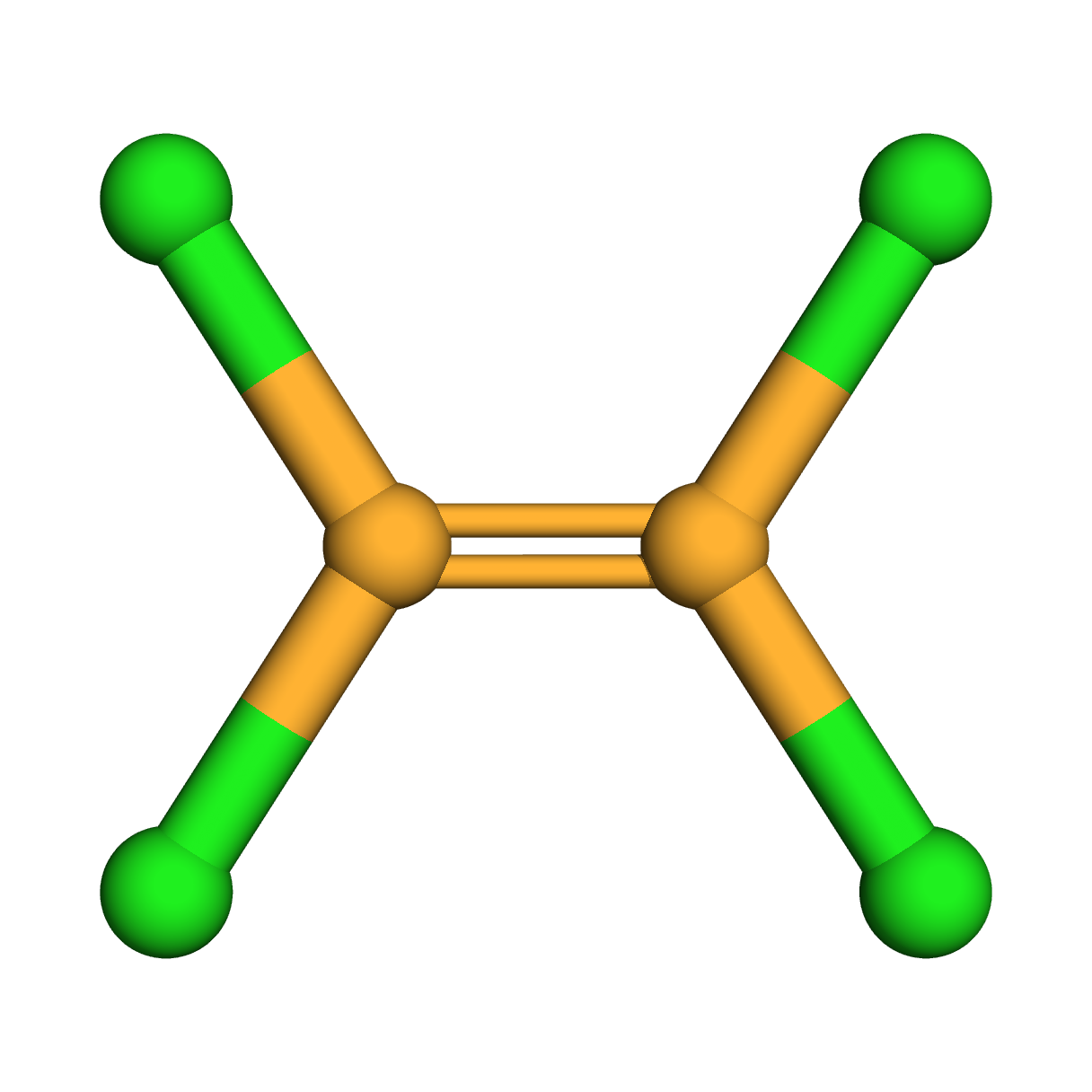
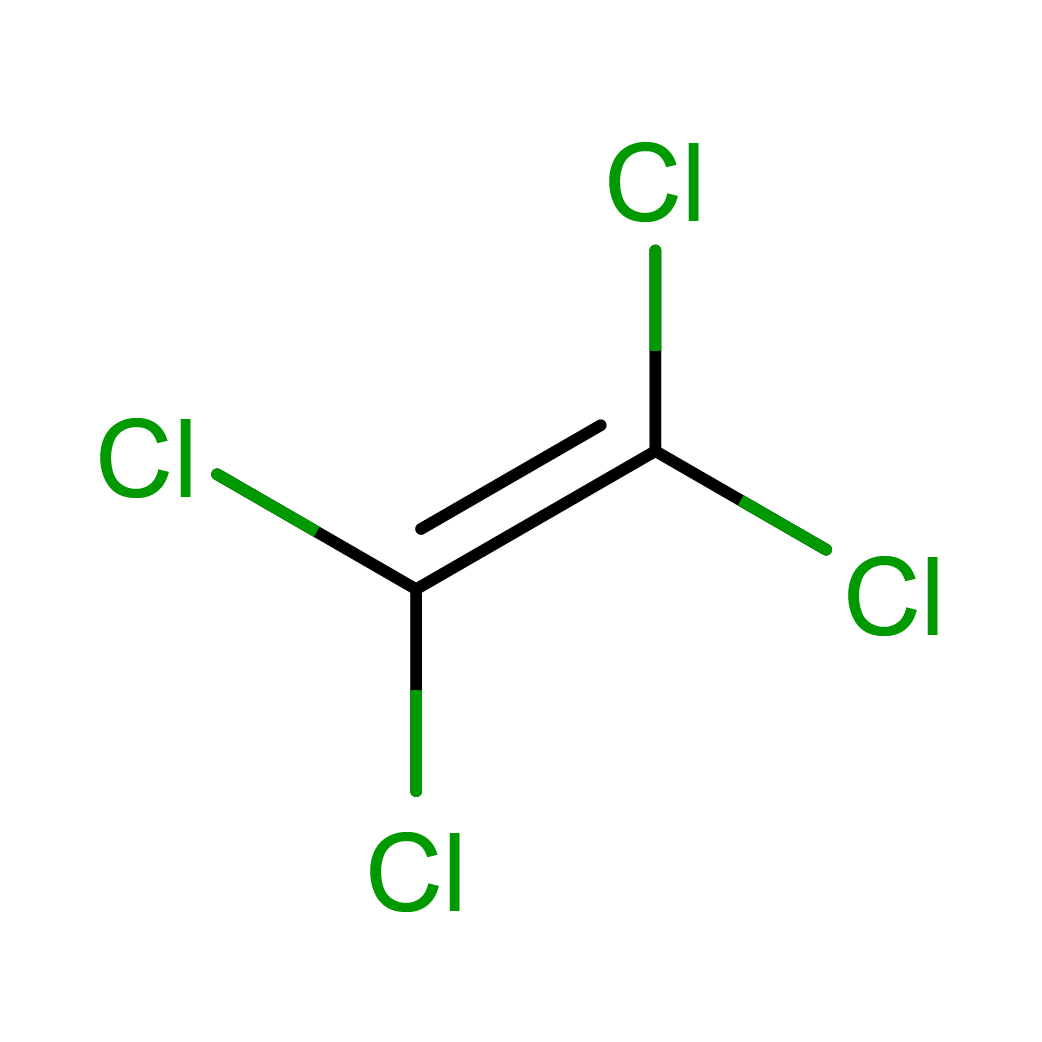
perchloroethylene
Synonyms: "tetrachloroethylene", "tetrachloroethene", "tetrachlorethylene", "ethylene tetrachloride"
Source: perchloroethylene is a chemical that is manufactured to be used in a wide range of commodities, including stain removal, adhesives, metal degreasing products and the manufacturing industry.
Identifiers:
IUPAC Name: 1,1,2,2-tetrachloroethene
CAS Number: 127-18-4
PubChem ID: 31373
InChiKey: CYTYCFOTNPOANT-UHFFFAOYSA-N
Canonical SMILES: C(=C(Cl)Cl)(Cl)Cl
Structural Properties:
Molecular Formula: C2Cl4
Molecular Weight: 165.833
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 6
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Carney EW, Thorsrud BA, Dugard PH, Zablotny CL. 2006. Developmental toxicity studies in Crl:CD (SD) rats following inhalation exposure to trichloroethylene and perchloroethylene. Birth Defects Res B Dev Reprod Toxicol 77(5):405-412.
Fredriksson A, Danielsson BR, Eriksson P. 1993. Altered behaviour in adult mice orally exposed to tri- and tetrachloroethylene as neonates. Toxicol Lett 66(1):13-19.
Honma T, Sudo A, Miyagawa M, Sato M, Hasegawa H. 1980. Effects of exposure to trichloroethylene and tetrachloroethylene on the contents of acetylcholine, dopamine, norepinephrine and serotonin in rat brain. Ind Health 18(4):171-178.
Shafer TJ, Bushnell PJ, Benignus VA, Woodward JJ. 2005. Perturbation of voltage-sensitive Ca2+ channel function by volatile organic solvents. J Pharmacol Exp Ther 315(3):1109-1118.
External Links

2D-structure
3D-structure