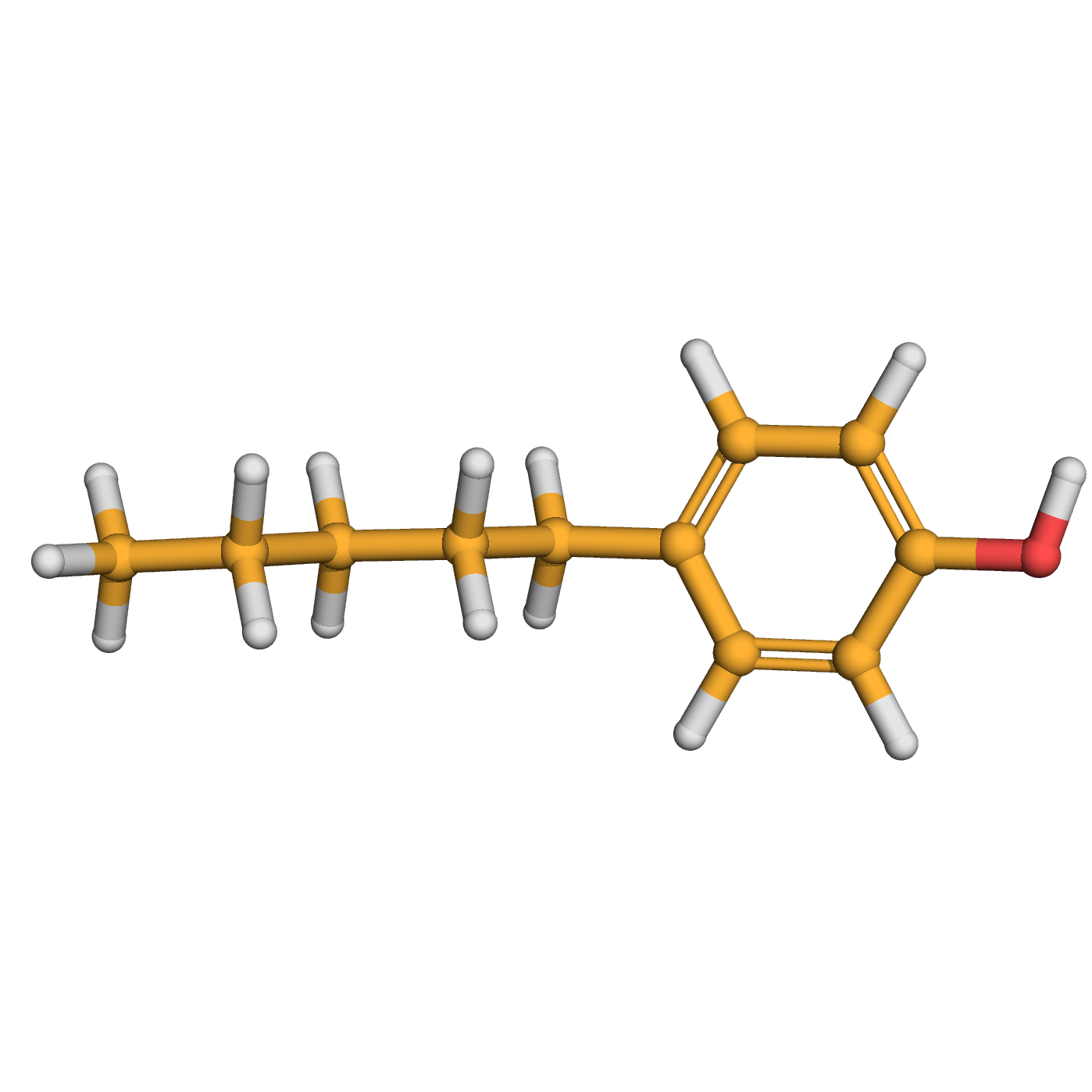
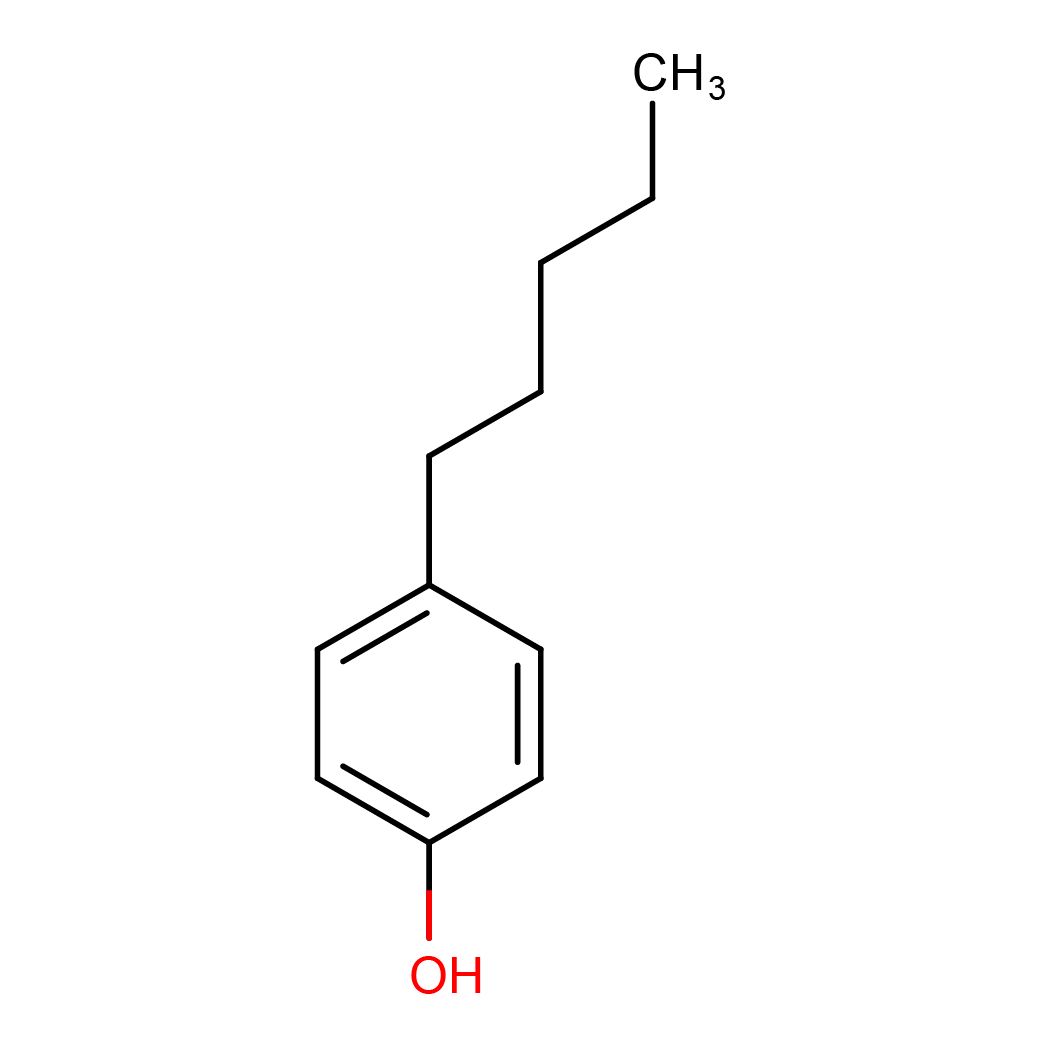
4-n-pentylphenol
Synonyms: "4-pentylphenol", "4-amylphenol", "4-pentyl-phenol", "4-n-amylphenol"
Source: 4-n-pentylphenol is an alkylphenol. The vast majority of alkylphenols are used to synthesize derivatives which have applications ranging from surfactants to pharmaceuticals.
Identifiers:
IUPAC Name: 4-pentylphenol
CAS Number: 14938-35-3
PubChem ID: 26975
InChiKey: ZNPSUQQXTRRSBM-UHFFFAOYSA-N
Canonical SMILES: CCCCCC1=CC=C(C=C1)O
Structural Properties:
Molecular Formula: C11H16O
Molecular Weight: 164.244
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 12
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. 2008. Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: Comparative study with 65 selected chemicals. Toxicol in Vitro 22(1):225-231.
Nakagomi M, Suzuki E, Usumi K, Saitoh Y, Yoshimura S, Nagao T, Ono H. 2001. Effects of endocrine disrupting chemicals on the microtubule network in Chinese hamster V79 cells in culture and in Sertoli cells in rats. Teratogenesis, Carcinogenesis & Mutagenesis 21(6):453-462.
Nishihara T, Nishikawa J, Kanayama T, Dakeyama F, Saito K, Imagawa M, Takatori S, Kitagawa Y, Hori S, Utsumi H. 2000. Estrogenic activities of 517 chemicals by yeast two-hybrid assay. Journal of Health Science 46(4):282-298.
Schultz TW, Sinks GD, Cronin MTD. 2000. Effect of substituent size and dimensionality on potency of phenolic xenoestrogens evaluated with a recombinant yeast assay. Environ Toxicol Chem 19(11):2637-2642.
External Links

2D-structure
3D-structure