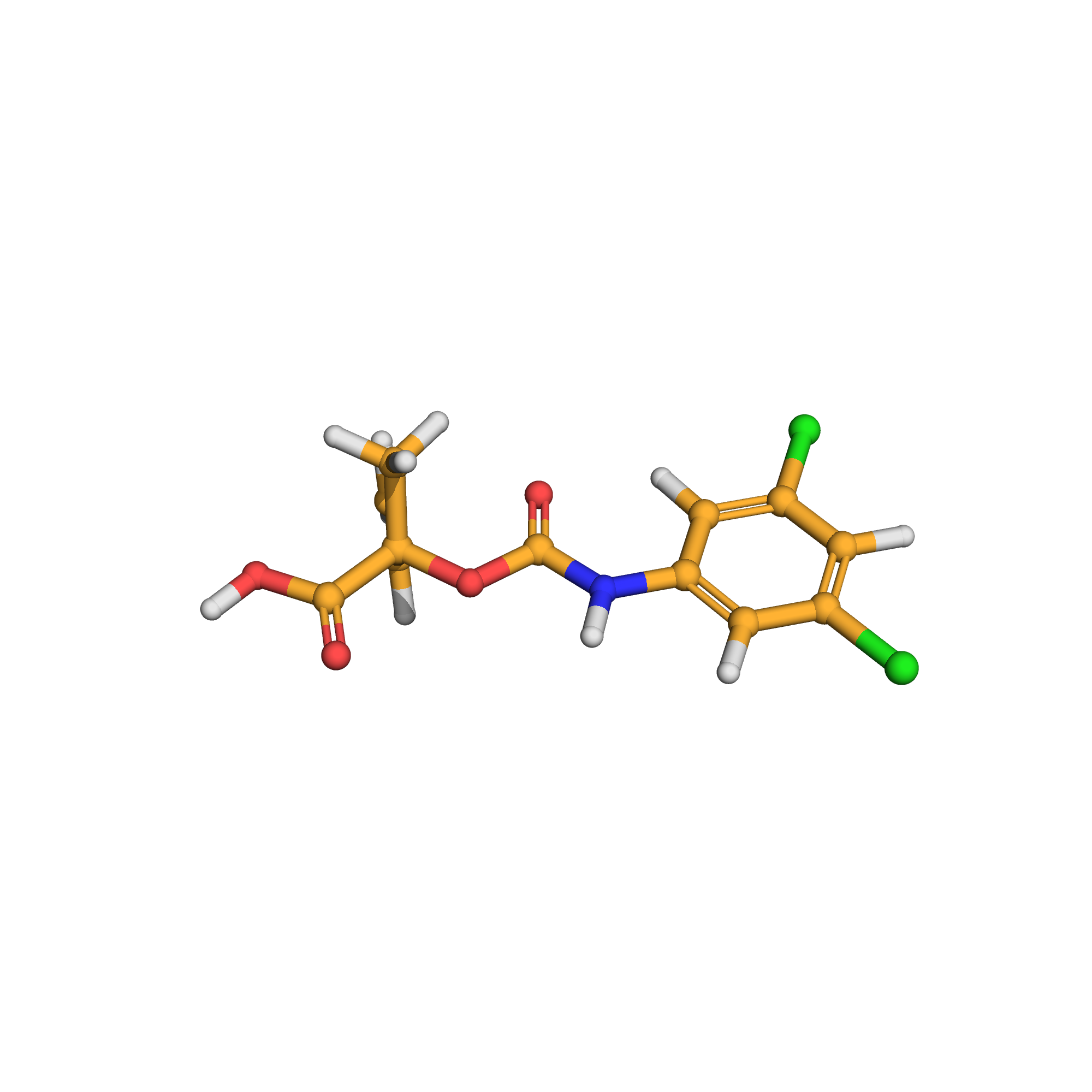
vinclozolin metabolite M1
Synonyms: "2-(((3,5-dichlorophenyl)carbamoyl)oxy)-2-methyl-3-butenoic acid ", "2-DCO-2-Me-butenoate", "2-[(3,5-dichlorophenyl)carbamoyloxy]-2-methylbut-3-enoic acid", "2-((((3,5-Dichlorophenyl)amino)carbonyl)oxy)-2-methyl-3-butenoic acid"
Source: vinclozolin metabolite M1 is one of the metabolites of the fungicide vinclozolin.
Identifiers:
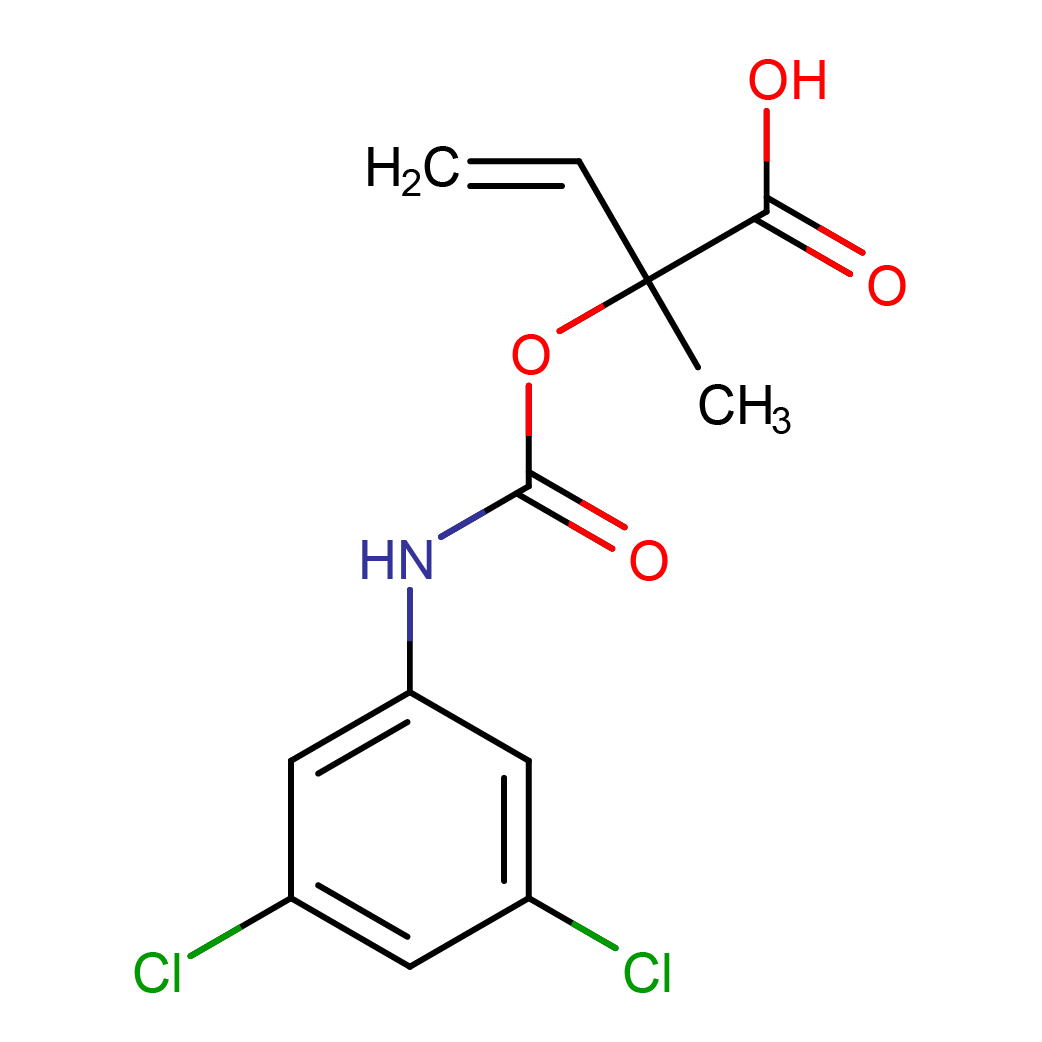
IUPAC Name: 2-[(3,5-dichlorophenyl)carbamoyloxy]-2-methylbut-3-enoic acid
CAS Number: 119209-27-7
PubChem ID: 119359
InChiKey: KTXGWKXVQGCPAR-UHFFFAOYSA-N
Canonical SMILES: CC(C=C)(C(=O)O)OC(=O)NC1=CC(=CC(=C1)Cl)Cl
Structural Properties:
Molecular Formula: C12H11Cl2NO4
Molecular Weight: 304.126
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 4
Number of atoms different from hydrogen: 19
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Laws SC, Carey SA, Kelce WR, Cooper RL, Gray LE. 1996. Vinclozolin does not alter progesterone receptor (PR) function in vivo despite inhibition of PR binding by its metabolites in vitro. Toxicology 112(3):173-182.
Wong CI, Kelce WR, Sar M, Wilson EM. 1995. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem 270(34):19998-20003.
External Links


2D-structure
3D-structure