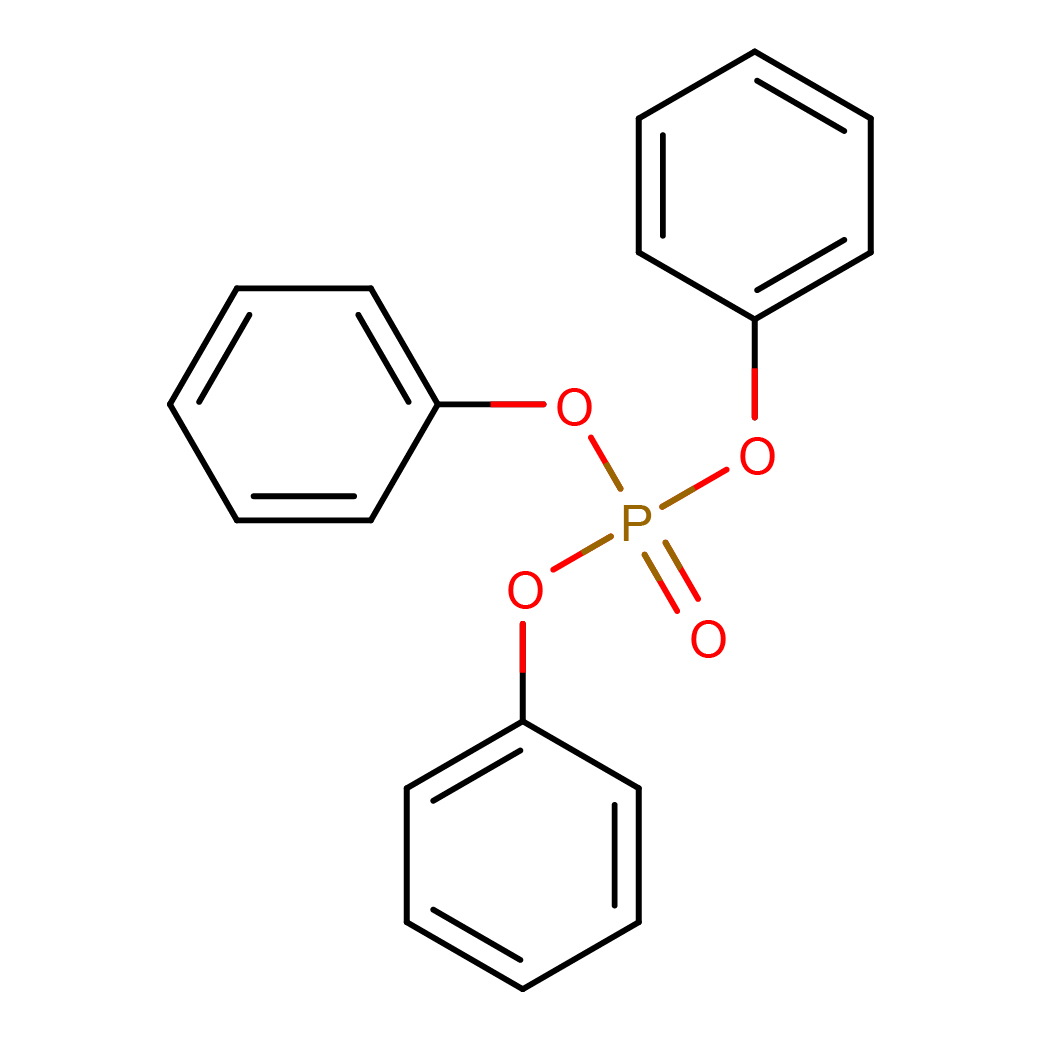
triphenyl phosphate
Synonyms: "disflamoll TP", "celluflex TPP", "phosflex TPP", "triphenoxyphosphine oxide", "phosphoric acid", "triphenyl ester", "triphenylphosphate"
Source: triphenyl phosphate is widely used as a flame retardant in phenolic and phenylene-oxide-based resins for the manufacture of electrical and automobile components. Triphenyl phosphate is also used as a non-flammable plasticizer in cellulose acetate for photographic films, and as a component of hydraulic fluids and lubricant oils.
Identifiers:
IUPAC Name: triphenyl phosphate
CAS Number: 115-86-6
PubChem ID: 8289
InChiKey: XZZNDPSIHUTMOC-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C(C=C1)OP(=O)(OC2=CC=CC=C2)OC3=CC=CC=C3
Structural Properties:
Molecular Formula: C18H15O4P
Molecular Weight: 326.283
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 1
Number of atoms different from hydrogen: 23
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Morisseau C, Merzlikin O, Lin A, He G, Feng W, Padilla I, Denison MS, Pessah IN, Hammock BD. 2009. Toxicology in the fast lane: application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ Health Perspect 117(12):1867-1872.
External Links



2D-structure
3D-structure