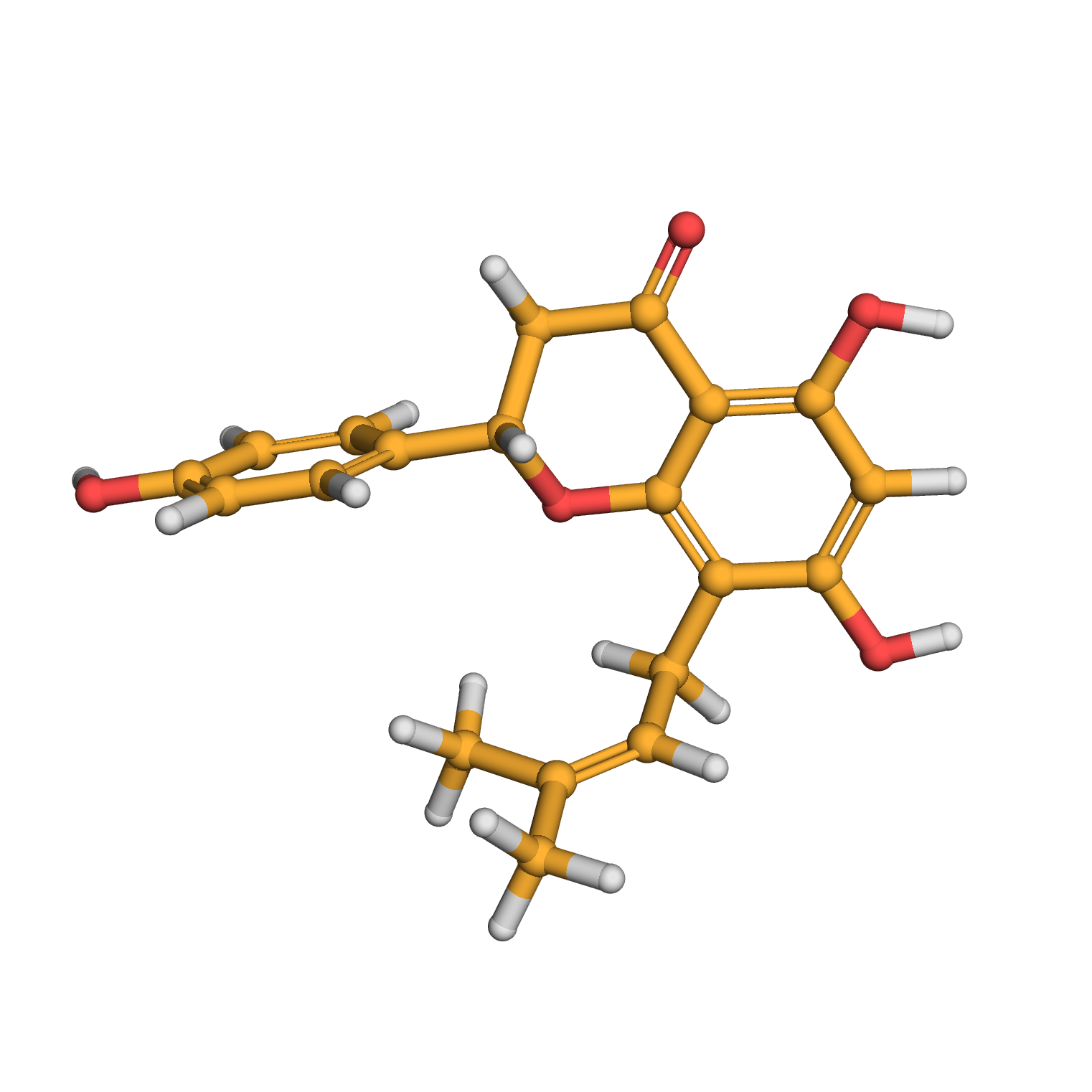
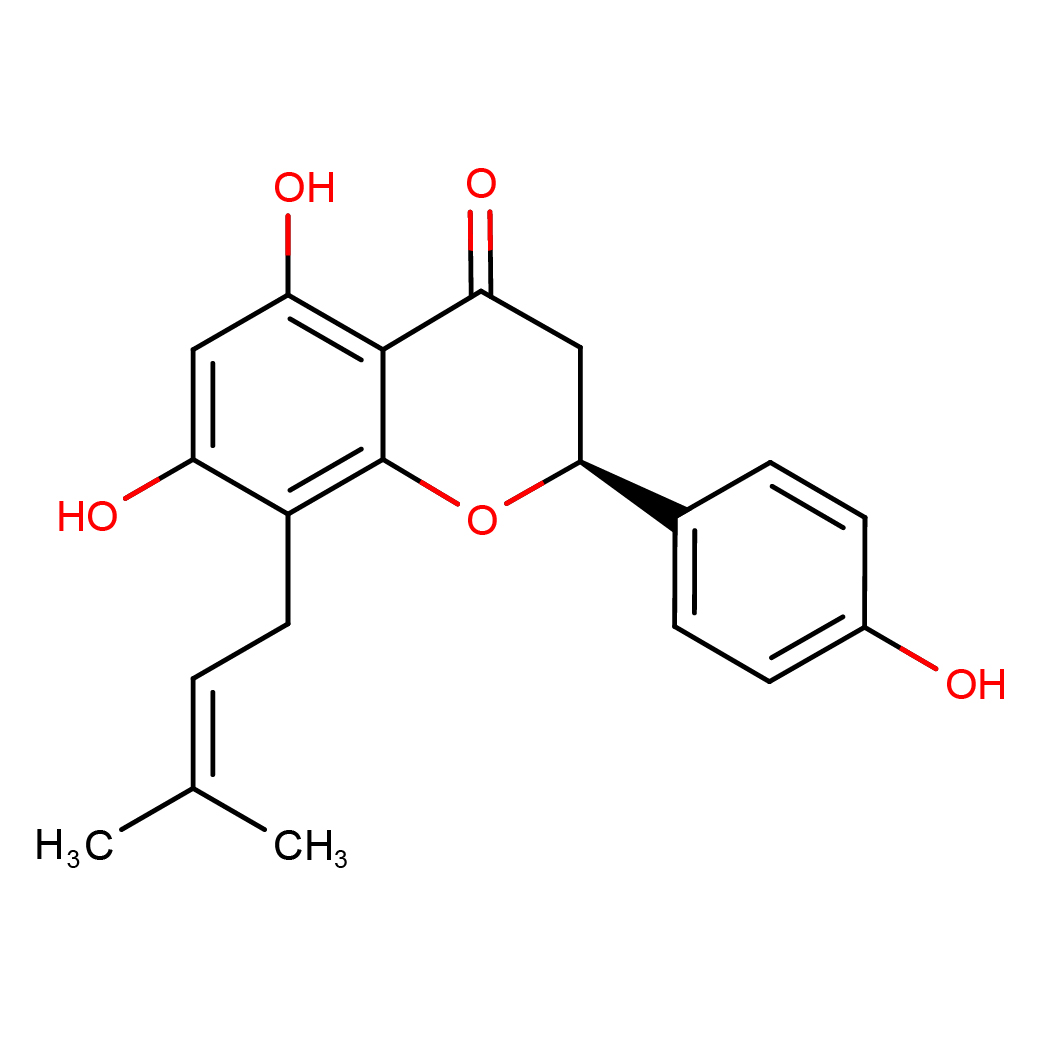
8-prenylnaringenin
Synonyms: "sophoraflavanone B", "(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-en-1-yl)-2,3-dihydro-4H-chromen-4-one", "flavaprenin"
Source: 8-prenylnaringenin is a prenylflavonoid and a potent plant-derived phytoestrogen.
Identifiers:
IUPAC Name: (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one
CAS Number: 53846-50-7
PubChem ID: 480764
InChiKey: LPEPZZAVFJPLNZ-SFHVURJKSA-N
Canonical SMILES: CC(=CCC1=C(C=C(C2=C1OC(CC2=O)C3=CC=C(C=C3)O)O)O)C
Structural Properties:
Molecular Formula: C20H20O5
Molecular Weight: 340.370
Pharmacophore Features:
Number of bond donors: 3
Number of bond acceptors: 5
Number of atoms different from hydrogen: 25
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Coldham NG, Sauer MJ. 2001. Identification, quantitation and biological activity of phytoestrogens in a dietary supplement for breast enhancement. Food & Chemical Toxicology 39(12):1211-1224.
Izzo G, Soder O, Svechnikov K. 2011. The prenylflavonoid phytoestrogens 8-prenylnaringenin and isoxanthohumol diferentially suppress steroidogenesis in rat Leydig cells in ontogenesis. J Appl Toxicol 31(6):589-594.
Milligan S, Kalita J, Pocock V, Heyerick A, De Cooman L, Rong H, De Keukeleire D. 2002. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction 123(2):235-242.
External Links


2D-structure
3D-structure