carbon tetrachloride
Synonyms: "tetrachloromethane", "carbona", "tetrasol", "carbon chloride", "perchloromethane", "vermoestricid", "benzinoform", "flukoids"
Source: carbon tetrachloride is found in air, water and soil. It was used in the production of refrigeration fluid and propellants for aerosol cans, as a pesticide, as a cleaning fluid and degreasing agent, in fire extinguishers, and in spot removers. Because of its harmful effects, these uses are now banned and it is only used in some industrial applications.
Identifiers:
IUPAC Name: tetrachloromethane
CAS Number: 56-23-5
PubChem ID: 5943
InChiKey: VZGDMQKNWNREIO-UHFFFAOYSA-N
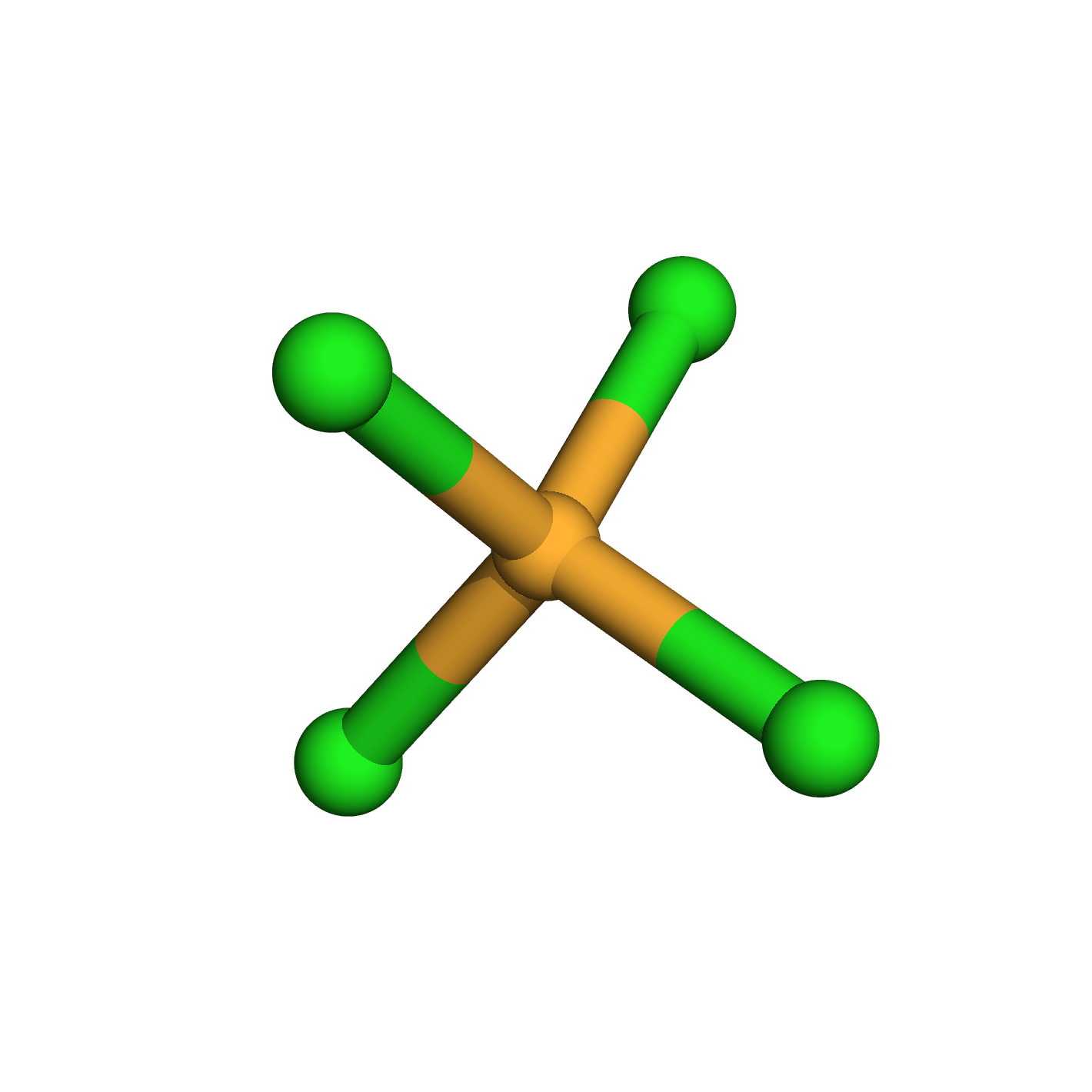
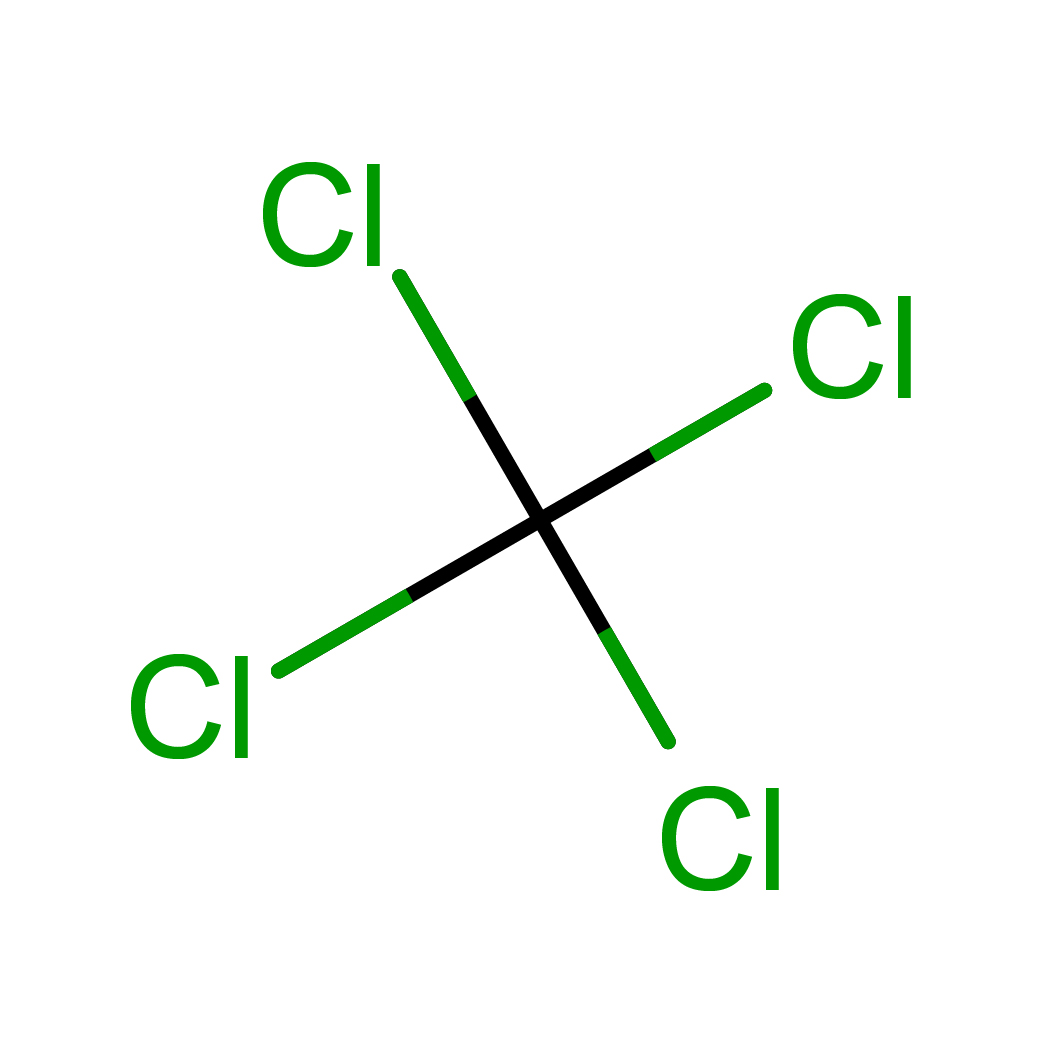
Canonical SMILES: C(Cl)(Cl)(Cl)Cl
Structural Properties:
Molecular Formula: CCl4
Molecular Weight: 153.823
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 0
Number of atoms different from hydrogen: 5
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Brogan WC, Eacho PI, Hinton DE, Colby HD. 1984. Effect of carbon tetrachloride on adrenocortical structure and function in guinea pigs. Toxicol Appl Pharmacol 75(1):118-127.
Colby HD, Purcell H, Kominami S, Takemori S, Kossor DC. 1994. Adrenal activation of carbon tetrachloride: Role of microsomal P450 isozymes. Toxicology 94(1-3):31-40.
External Links


2D-structure
3D-structure