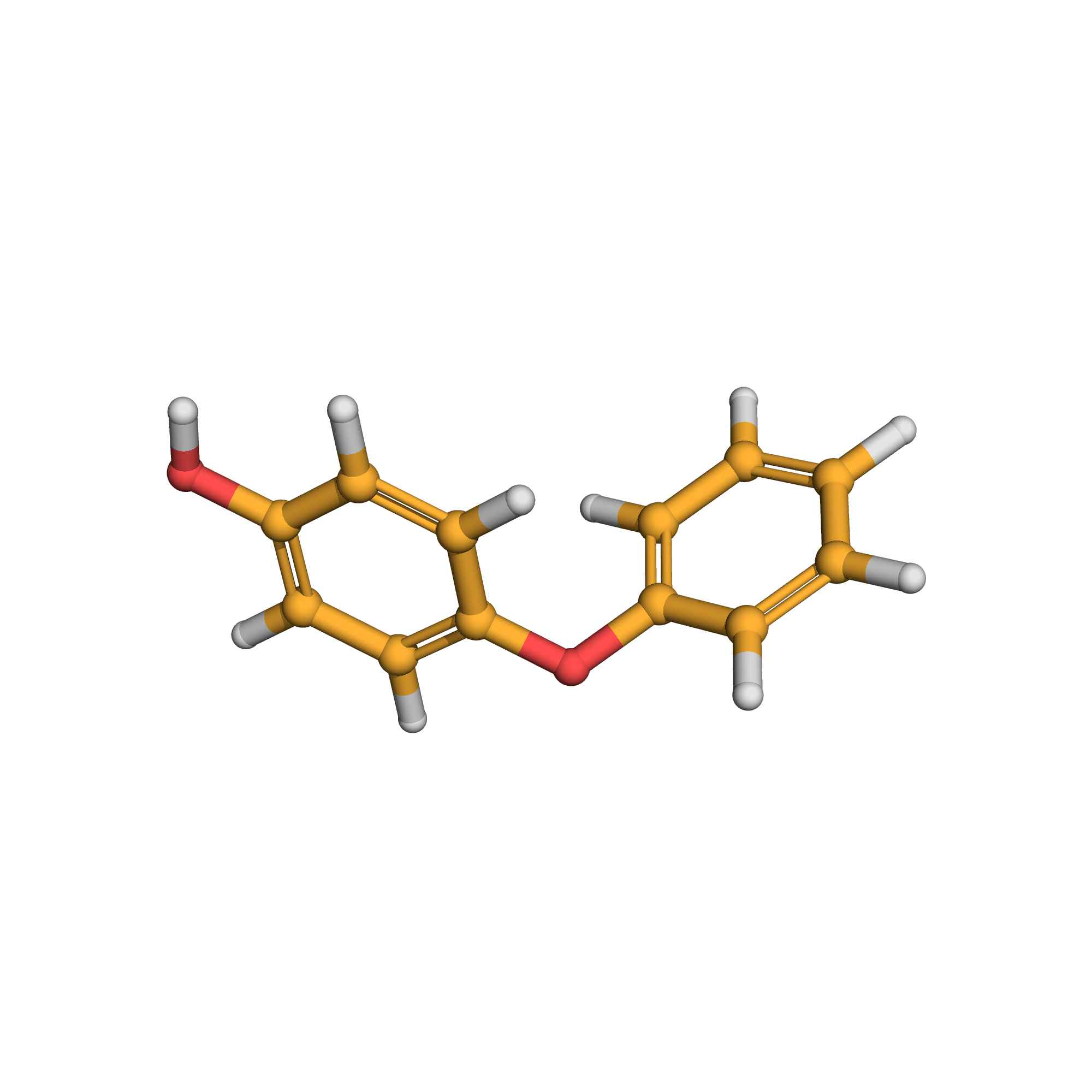
4-phenoxyphenol
Synonyms: "p-phenoxyphenol", "p-hydroxydiphenyl ether", "4-hydroxydiphenyl ether", "hydroquinone monophenyl ether", "4-(phenyloxy)phenol"
Source: 4-phenoxyphenol is a by-product of pesticide decomposition, and persistent cause of pollution.
Identifiers:
IUPAC Name: 4-phenoxyphenol
CAS Number: 831-82-3
PubChem ID: 13254
InChiKey: ZSBDGXGICLIJGD-UHFFFAOYSA-N
Canonical SMILES: C1=CC=C(C=C1)OC2=CC=C(C=C2)O
Structural Properties:
Molecular Formula: C12H10O2
Molecular Weight: 186.207
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 1
Number of atoms different from hydrogen: 14
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman Å,, Lemmen JG, van der Burg B, Brouwer A. 2001. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect 109(4):399-407, DOI: 10.2307/3454900.
Schultz TW, Sinks GD, Cronin MTD. 2000. Effect of substituent size and dimensionality on potency of phenolic xenoestrogens evaluated with a recombinant yeast assay. Environ Toxicol Chem 19(11):2637-2642.
External Links


2D-structure
3D-structure