triadimefon
Synonyms: "bayleton", "azocene", "triadimefone", "fenxiunin", "haleton", "acizol", "adifon", "amiral"
Source: triadimefon is a widely used fungicide.
Identifiers:
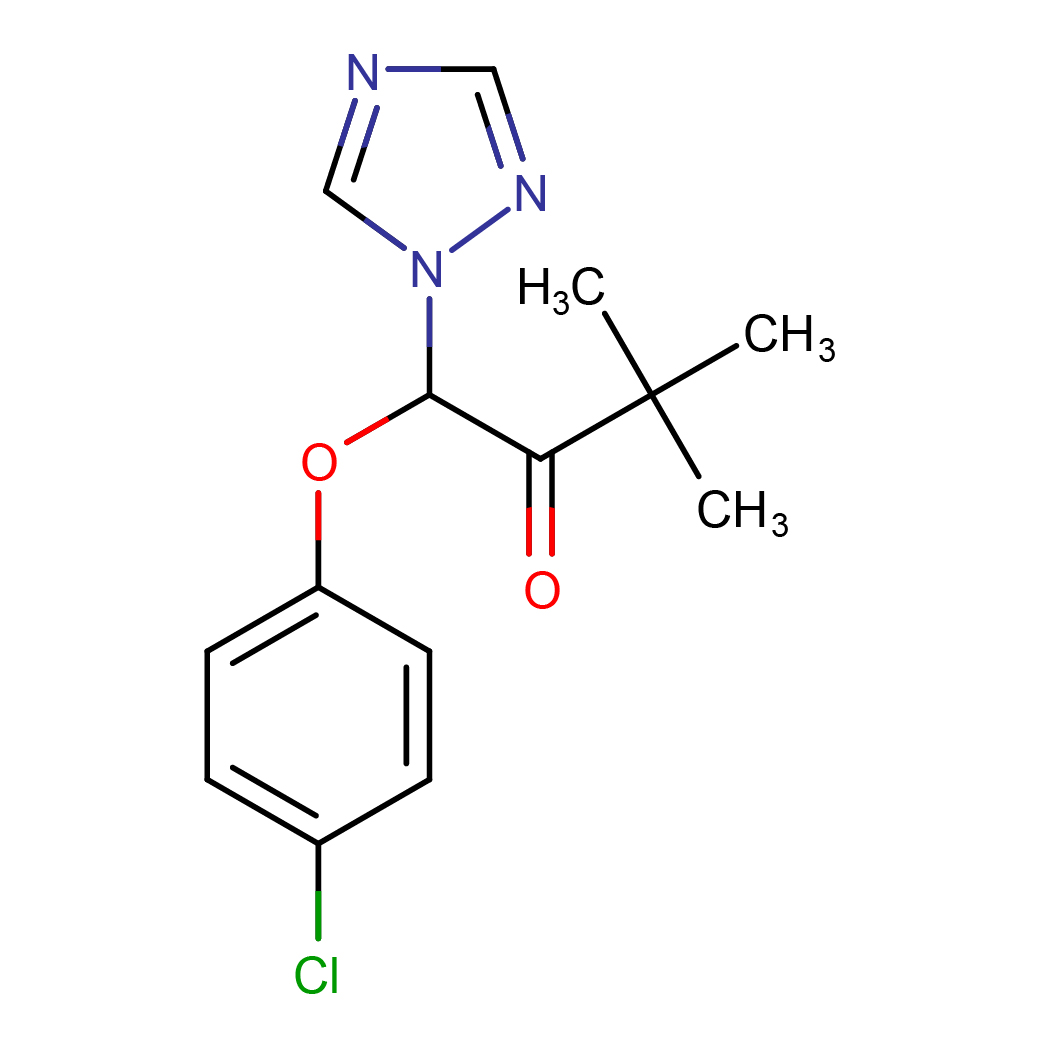
IUPAC Name: 1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-one
CAS Number: 43121-43-3
PubChem ID: 39385
InChiKey: WURBVZBTWMNKQT-UHFFFAOYSA-N
Canonical SMILES: CC(C)(C)C(=O)C(N1C=NC=N1)OC2=CC=C(C=C2)Cl
Structural Properties:
Molecular Formula: C14H16ClN3O2
Molecular Weight: 293.749
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 4
Number of atoms different from hydrogen: 20
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hurley PM, Hill RN, Whiting RJ. 1998. Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents [review]. Environ Health Perspect 106(8):437-445.
Petit F, Le Goff P, Cravedi JP, Valotaire Y, Pakdel F. 1997. Two complementary bioassays for screening the estrogenic potency of xenobiotics: recombinant yeast for trout estrogen receptor and trout hepatocyte cultures. J Mol Endocrinol 19(3):321-335.
Vinggaard AM, Breinholt V, Larsen JC. 1999. Screening of selected pesticides for oestrogen receptor activation in vitro. Food Additives & Contaminants 16(12):533-542.
Vinggaard AM, Hnida C, Breinholt V, Larsen JC. 2000. Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol in Vitro 14(3):227-234.
External Links


2D-structure
3D-structure