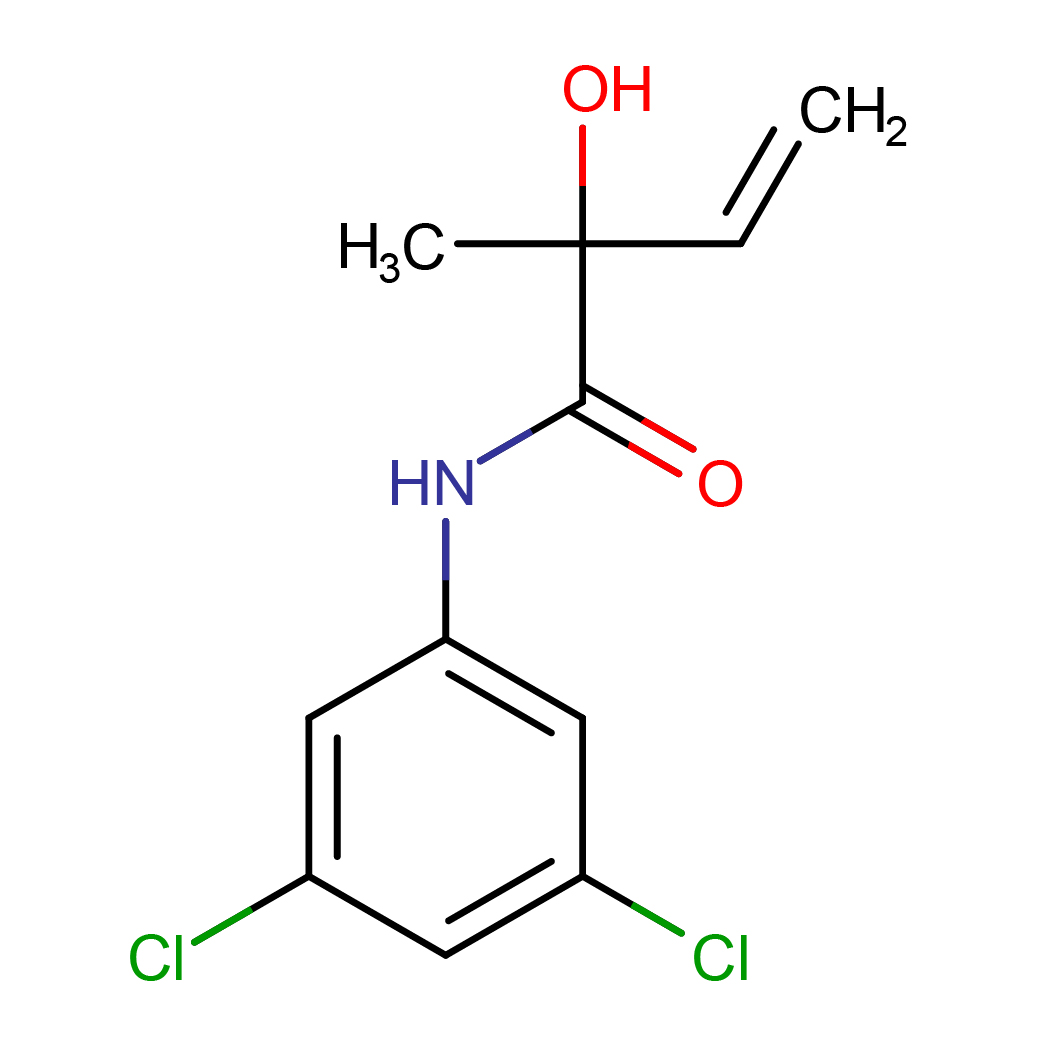
3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide
Synonyms: "N-(3,5-dichlorophenyl)-2-hydroxy-2-methyl-3-butenamide", "3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide", "n-(3,5-dichlorophenyl)-2-hydroxy-2-methylbut-3-enamide", "2-hydroxy-2-methyl-N-(3,5-dichlorophenyl)-3-butenamide", "N-(3,5-dichlorophenyl)-2-hydroxy-2-methyl-but-3-enamide", "vinclozolin M2", "M2".
Source: Vinclozolin M2 is a dicarboximide fungicide.
Identifiers:
IUPAC Name: N-(3,5-dichlorophenyl)-2-hydroxy-2-methylbut-3-enamide
CAS Number: 83792-61-4
PubChem ID: 115209
InChiKey: FBYYIBNYONAZCU-UHFFFAOYSA-N
Canonical SMILES: CC(C=C)(C(=O)NC1=CC(=CC(=C1)Cl)Cl)O
Structural Properties:
Molecular Formula: C11H11Cl2NO2
Molecular Weight: 260.113
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 2
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Laws SC, Carey SA, Kelce WR, Cooper RL, Gray LE. 1996. Vinclozolin does not alter progesterone receptor (PR) function in vivo despite inhibition of PR binding by its metabolites in vitro. Toxicology 112(3):173-182. DOI: 10.1016/0300-483X(96)03354-9. URL: https://www.sciencedirect.com/science/article/pii/0300483X96033549.
Wong CI, Kelce WR, Sar M, Wilson EM. 1995. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem 270(34):19998-20003. DOI: 10.1074/jbc.270.34.19998 . URL: http://www.jbc.org/content/270/34/19998.long.
External Links


2D-structure
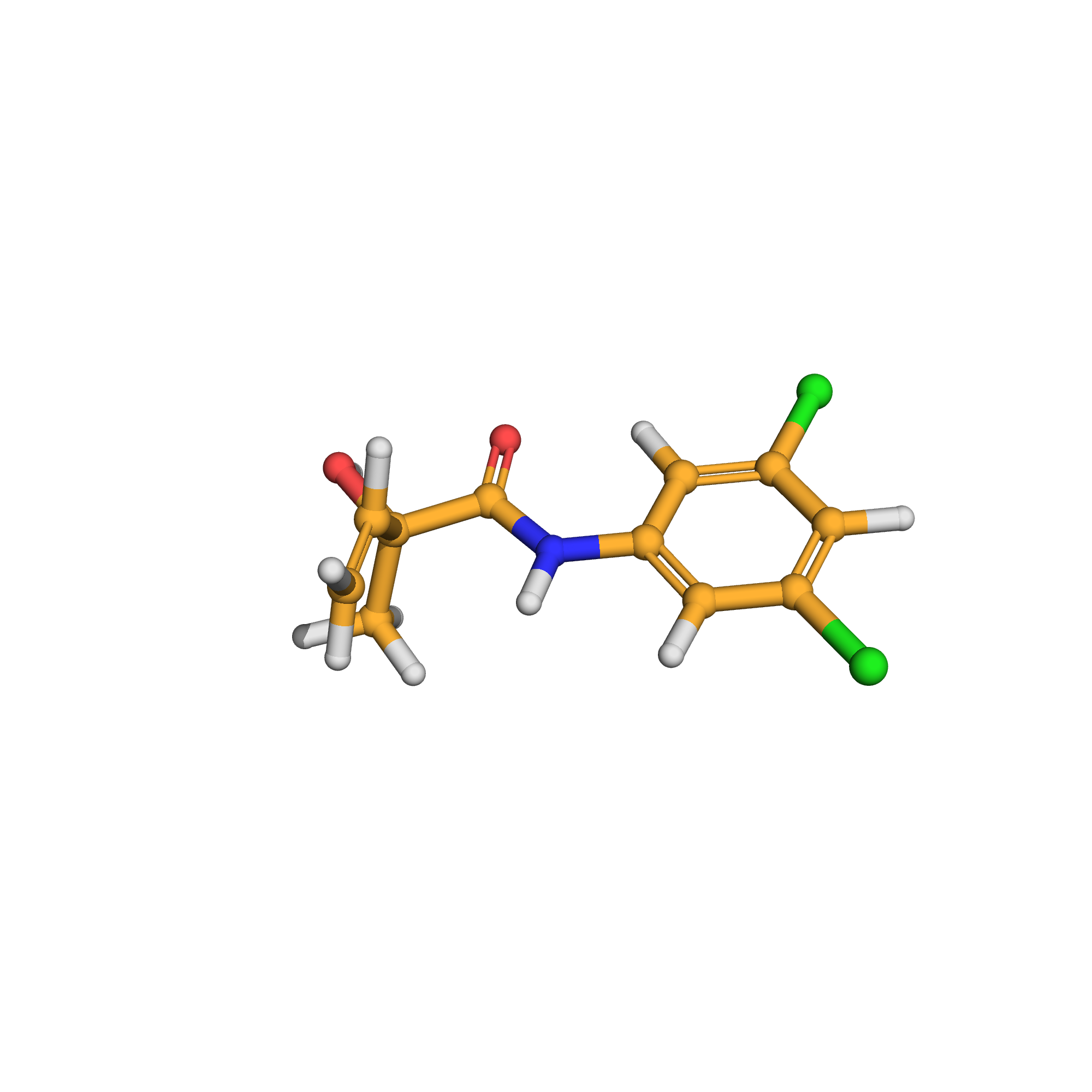
3D-structure