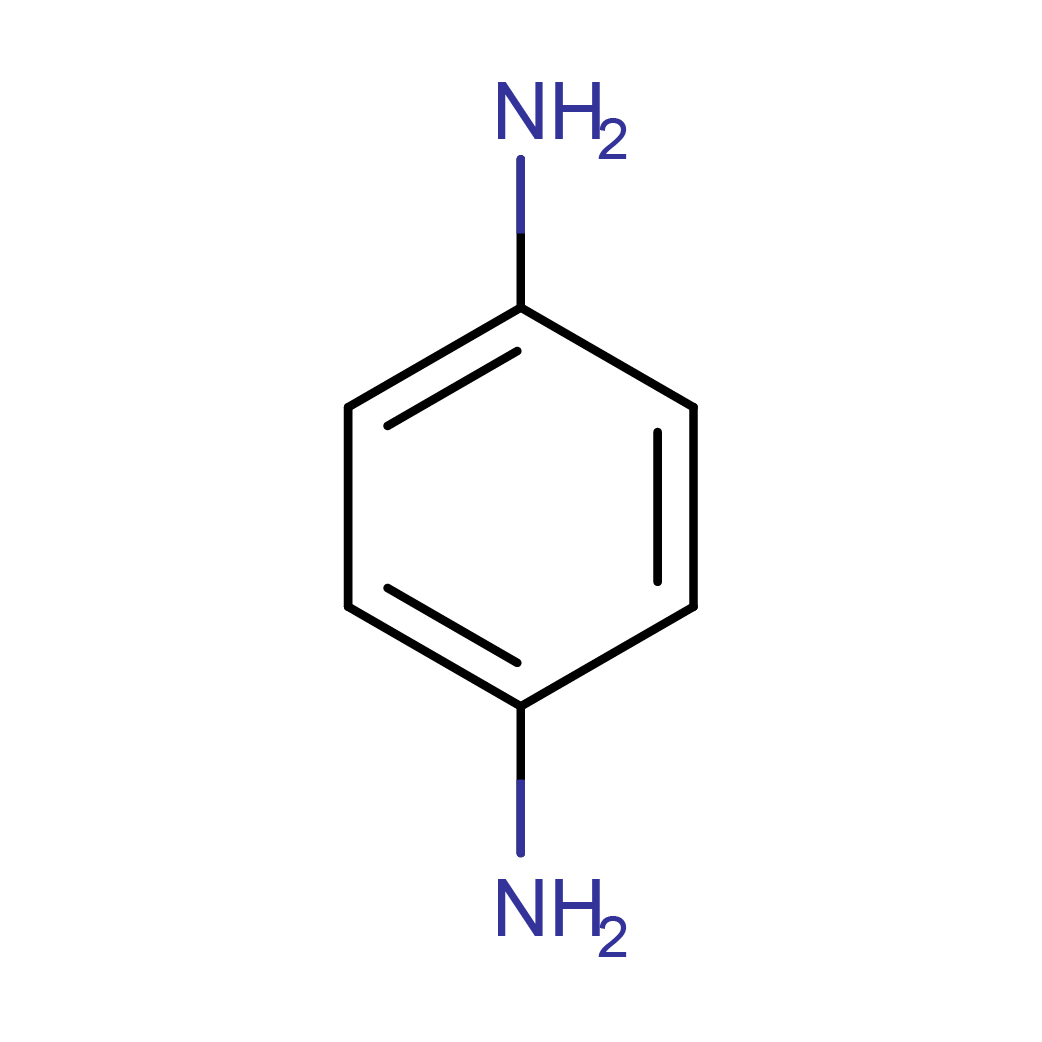
p-phenylenediamine
Synonyms: "p-phenylenediamine", "benzene-1,4-diamine", "1,4-benzenediamine", "1,4-diaminobenzene", "1,4-phenylenediamine", "4-aminoaniline", "para-phenylenediamine", "p-aminoaniline", "p-diaminobenzene", "paraphenylenediamine", "4-phenylenediamine", "p-benzenediamine", "Futramine D", "Fouramine D", "Fourrine D", "Fur Yellow", "Benzofur D", "Santoflex IC", "Santoflex LC", "Developer PF", "Tertral D", "Pelagol D", "Peltol D", "Durafur Black R", "Pelagol DR", "Pelagol Grey D", "Fourrine 1", "Furro D", "Ursol D", "Zoba Black D", "Renal PF", "Developer 13", "Nako H", "BASF ursol D", "Oxidation base 10", "Fenyle","Orsin".
Source: p-Phenylenediamine is primarily used as a dye intermediate and as a dye, including hair dye formulations
Identifiers:
IUPAC Name: benzene-1,4-diamine
CAS Number: 106-50-3
PubChem ID: 7814
InChiKey: CBCKQZAAMUWICA-UHFFFAOYSA-N
Canonical SMILES: C1=CC(=CC=C1N)N
Structural Properties:
Molecular Formula: C6H8N2
Molecular Weight: 108.144
Pharmacophore Features:
Number of bond donors: 2
Number of bond acceptors: 2
Number of atoms different from hydrogen: 8
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Bharali, M.K. and Dutta, K., 2012. Testicular toxicity of para-phenylenediamine after subchronic topical application in rat. International journal of environmental health research, 22(3), pp.270-278. DOI: https://doi.org/10.1080/09603123.2011.634388. URL: https://www.tandfonline.com/doi/abs/10.1080/09603123.2011.634388.
External Links



2D-structure
3D-structure