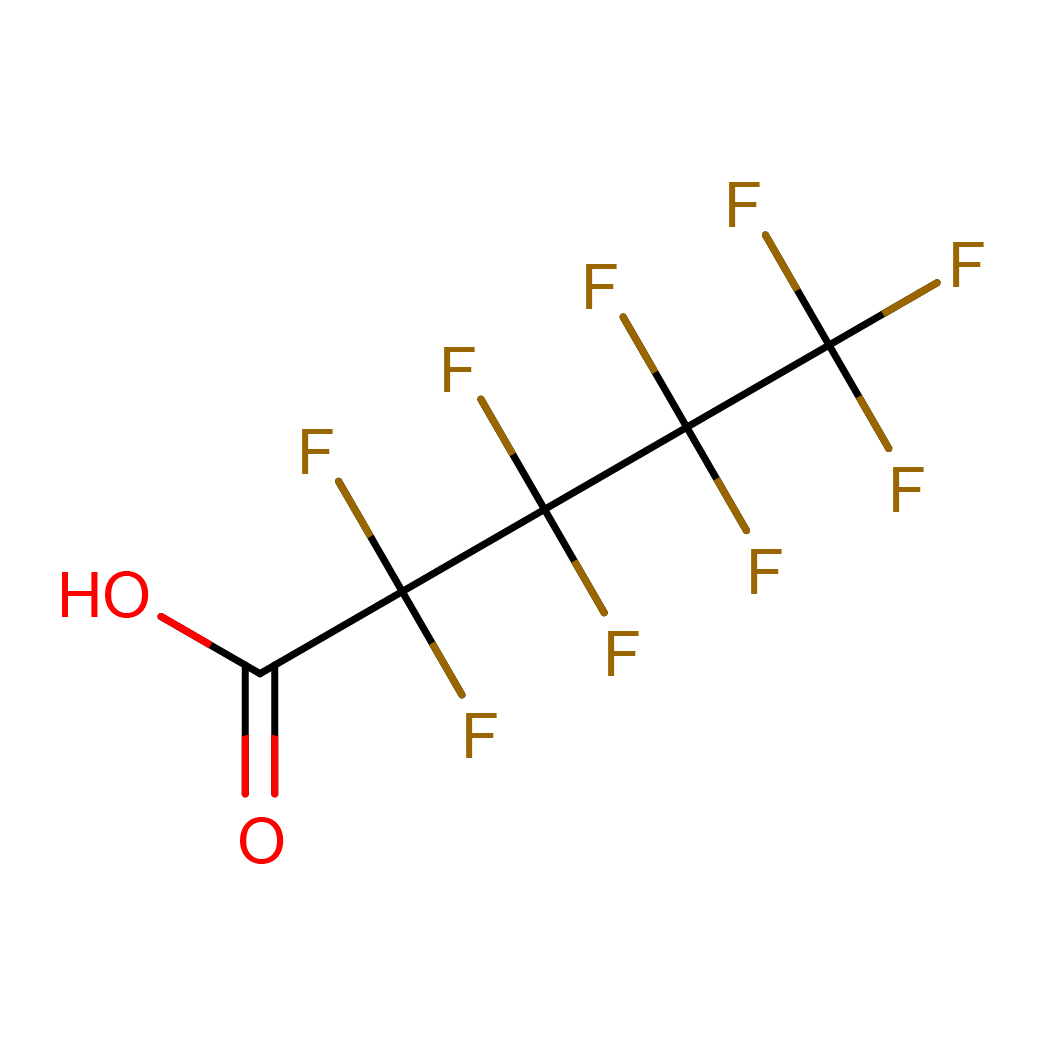
perfluorovaleric acid
Synonyms: "perfluoropentanoic acid", "perfluorovaleric acid", "nonafluoropentanoic acid", "nonafluorovaleric acid", "2,2,3,3,4,4,5,5,5-nonafluoropentanoic acid", "n-perfluoropentanoic acid", "nonafluoro-1-pentanoic acid", "perfluoro-n-pentanoic acid", "2,2,3,3,4,4,5,5,5-nonafluoro-pentanoic acid".
Source: perfluorovaleric acid is a member of a class of perfluorinated alkanoic acids commonly used as lubricants, plasticizers, wetting agents and corrosion, as well as emulsifiers and surfactants in fluoropolymer manufacturing.
Identifiers:
IUPAC Name: 2,2,3,3,4,4,5,5,5-nonafluoropentanoic acid
CAS Number: 2706-90-3
PubChem ID: 75921
InChiKey: CXZGQIAOTKWCDB-UHFFFAOYSA-N
Canonical SMILES: C(=O)(C(C(C(C(F)(F)F)(F)F)(F)F)(F)F)O
Structural Properties:
Molecular Formula: C5HF9O2
Molecular Weight: 264.047
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 11
Number of atoms different from hydrogen: 16
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Ishibashi H, Kim EY and Iwata H, 2011. Transactivation potencies of the Baikal seal (Pusa sibirica) peroxisome proliferator-activated receptor ? by perfluoroalkyl carboxylates and sulfonates: estimation of PFOA induction equivalency factors. Environ Sci Technol 45(7):3123-3130. DOI: 10.1021/es103748s. URL: https://www.ncbi.nlm.nih.gov/pubmed/21381677.
Li Y, Cheng Y, Xie Z and Zeng F. 2017. Perfluorinated alkyl substances in serum of the southern Chinese general population and potential impact on thyroid hormones. Scientific Reports 7:43380. DOI: 10.1038/srep43380. URL: https://www.ncbi.nlm.nih.gov/pubmed/28240244.
Rosenmai AK, Ahrens L, Godec T, Lundqvist J and Oskarsson A. 2017. Relationship between peroxisome proliferator?activated receptor alpha activity and cellular concentration of 14 perfluoroalkyl substances in HepG2 cells. J Appl Toxicol 38(2):219-226. DOI: 10.1002/jat.3515. URL: https://www.ncbi.nlm.nih.gov/pubmed/28857218.
Shah-Kulkarni, S., Kim, B.M., Hong, Y.C., Kim, H.S., Kwon, E.J., Park, H., Kim, Y.J. and Ha, E.H., 2016. Prenatal exposure to perfluorinated compounds affects thyroid hormone levels in newborn girls. Environment international, 94, pp.607-613. DOI: 10.1016/j.envint.2016.06.024. URL: http://www.sciencedirect.com/science/article/pii/S0160412016302422.
Wolf CJ, Schmid JE, Lau C and Abbott BD. 2012. Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPAR?) by perfluoroalkyl acids (PFAAs): further investigation of C4?C12 compounds. Reprod Toxicol 33(4):546-551. DOI: 10.1016/j.reprotox.2011.09.009. URL: https://www.ncbi.nlm.nih.gov/pubmed/22107727.
External Links





2D-structure
3D-structure