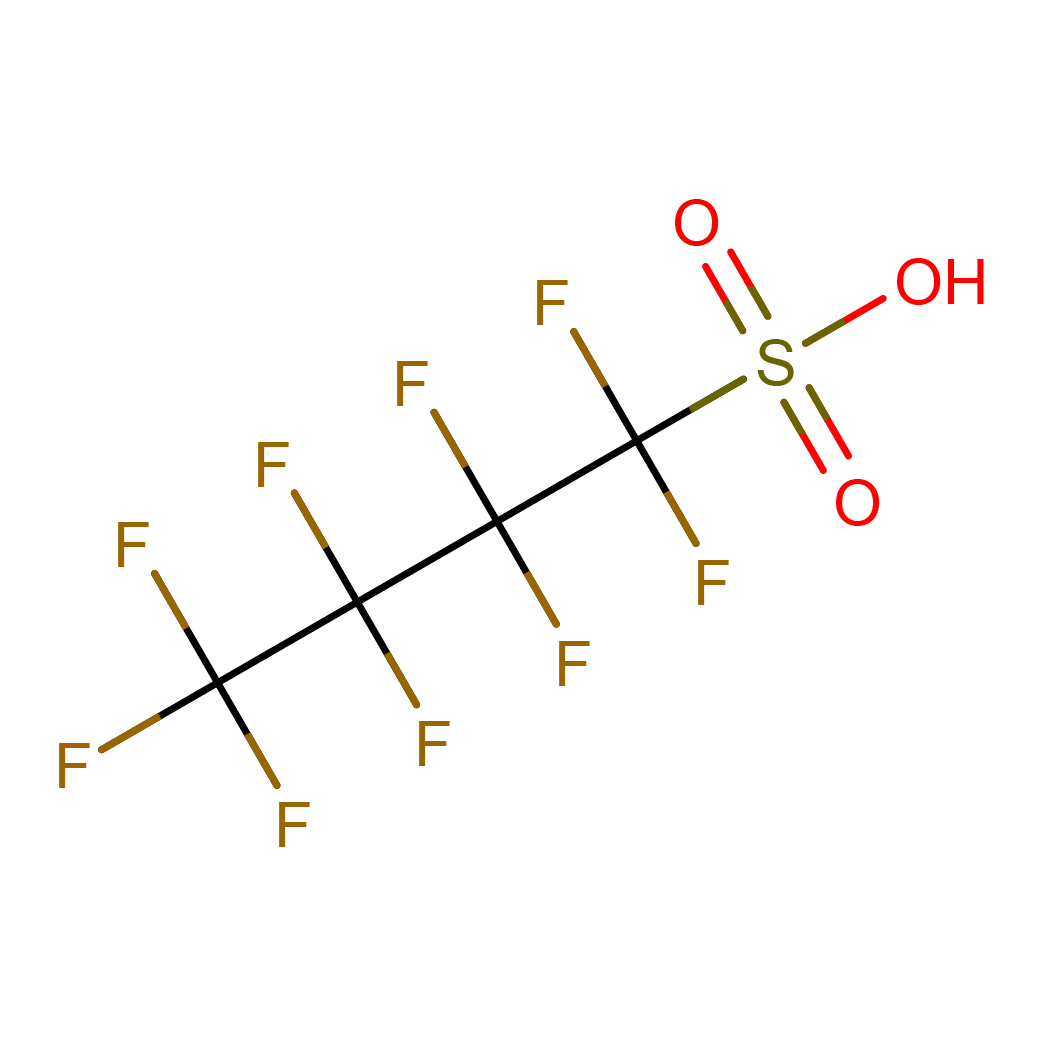
nonafluorobutanesulfonic acid
Synonyms: "375-73-5", "Nonafluorobutanesulfonic acid", "perfluorobutanesulfonic acid", "Nonafluoro-1-butanesulfonic acid", "1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonic acid", "1,1,2,2,3,3,4,4,4-Nonafluoro-1-butanesulfonic acid", "PFBS", "perfluorobutane sulfonic acid".
Source: nonafluorobutanesulfonic acid is a commercially available superstrong perfluoroalkyl acid, which is used during the manufactures of fluoropolymers.
Identifiers:
IUPAC Name: 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonic acid
CAS Number: 375-73-5
PubChem ID: 67815
InChiKey: JGTNAGYHADQMCM-UHFFFAOYSA-N
Canonical SMILES: C(C(C(F)(F)S(=O)(=O)O)(F)F)(C(F)(F)F)(F)F
Structural Properties:
Molecular Formula: C4HF9O3S
Molecular Weight: 300.095
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 12
Number of atoms different from hydrogen: 17
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Feng X, Cao X, Zhao S, Wang X, Hua X, Chen L and Chen L. 2017. Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring. Toxicological Sciences, 155(2):409-419. DOI: 10.1093/toxsci/kfw219. URL: https://www.ncbi.nlm.nih.gov/pubmed/27803384.
Gorrochategui E, Parez-Albaladejo E, Casas J, Lacorte S and Porte C. 2014. Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol Appl Pharmacol 277(2):124-130. DOI: 10.1016/j.taap.2014.03.012. URL: https://www.ncbi.nlm.nih.gov/pubmed/24680846.
Lou QQ, Zhang YF, Zhou Z, Shi YL, Ge YN, Ren DK, Xu HM, Zhao YX, Wei WJ and Qin ZF. 2013. Effects of perfluorooctanesulfonate and perfluorobutanesulfonate on the growth and sexual development of Xenopus laevis. Ecotoxicology 22(7):1133-1144. DOI: 10.1007/s10646-013-1100-y. URL: https://www.ncbi.nlm.nih.gov/pubmed/23907449.
Rosenmai AK, Ahrens L, Godec T, Lundqvist J and Oskarsson A. 2017. Relationship between peroxisome proliferator?activated receptor alpha activity and cellular concentration of 14 perfluoroalkyl substances in HepG2 cells. J Appl Toxicol 38(2):219-226. DOI: 10.1002/jat.3515. URL: https://www.ncbi.nlm.nih.gov/pubmed/28857218.
Vongphachan V, Cassone CG, Wu D, Chiu S, Crump D, Kennedy SW. 2011. Effects of perfluoroalkyl compounds on mRNA expression levels of thyroid hormone-responsive genes in primary cultures of avian neuronal cells. Toxicol Sci 120(2):392-402. DOI: 10.1093/toxsci/kfq395. URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3061477/.
External Links


2D-structure
3D-structure