leucomalachite green
Synonyms: "Leuco malachite green", "Malachite green leuco", "Malachite green leuco base", "4,4'-Bis(dimethylamino)triphenylmethane", "Tetramethyldiaminotriphenylmethane", "bis(p-dimethylaminophenyl)phenylmethane", "p,p'-benzylidenebis(N,N-dimethylaniline)", "N,N,N',N'-tetramethyl-4,4'-benzylidenedianiline", "4,4'-benzylidenebis(N,N-dimethylaniline)".
Source: Leucomalachite green is the primary metabolite of malachite green, which is an organic compound that is used as a dyestuff and as antimicrobial.
Identifiers:
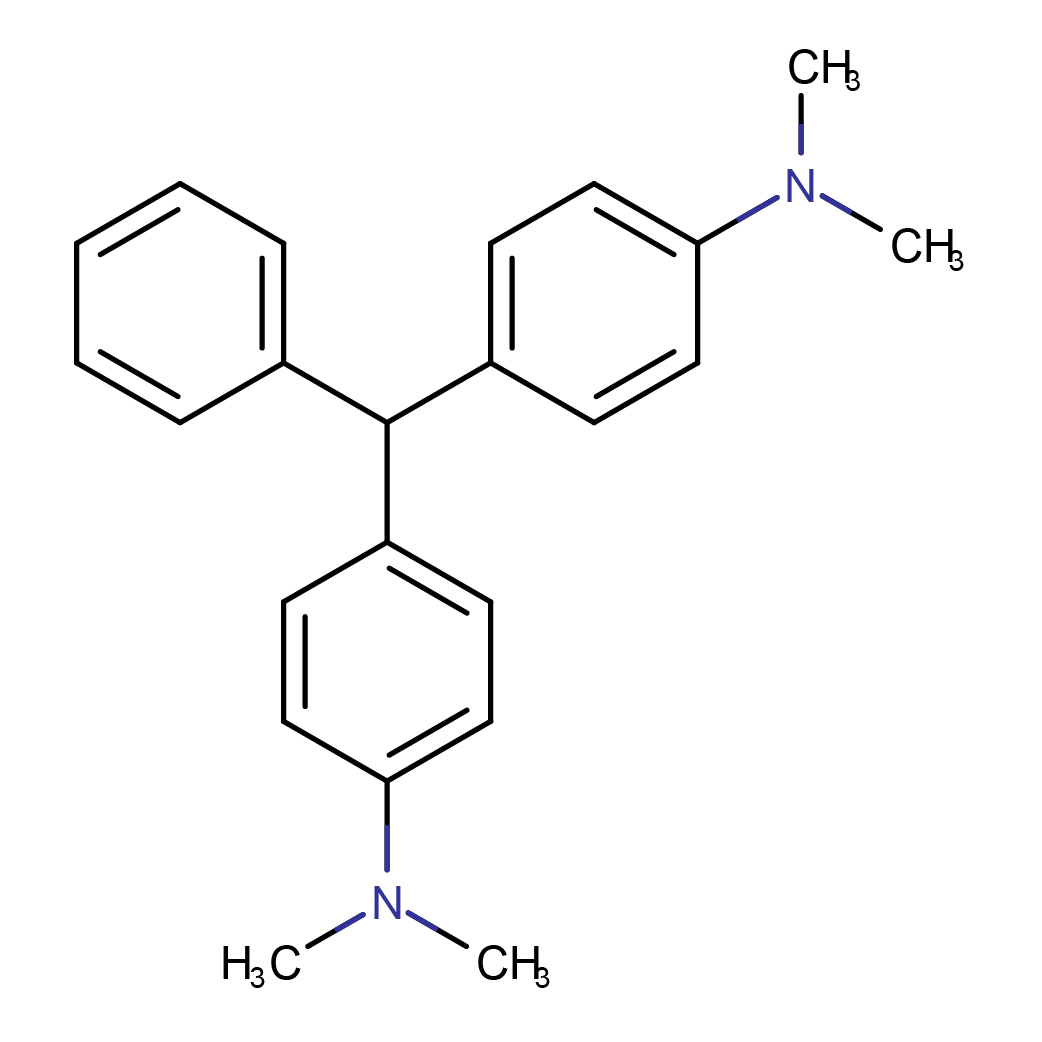
IUPAC Name: 4-[[4-(dimethylamino)phenyl]-phenylmethyl]-N,N-dimethylaniline
CAS Number: 129-73-7
PubChem ID: 67215
InChiKey: WZKXBGJNNCGHIC-UHFFFAOYSA-N
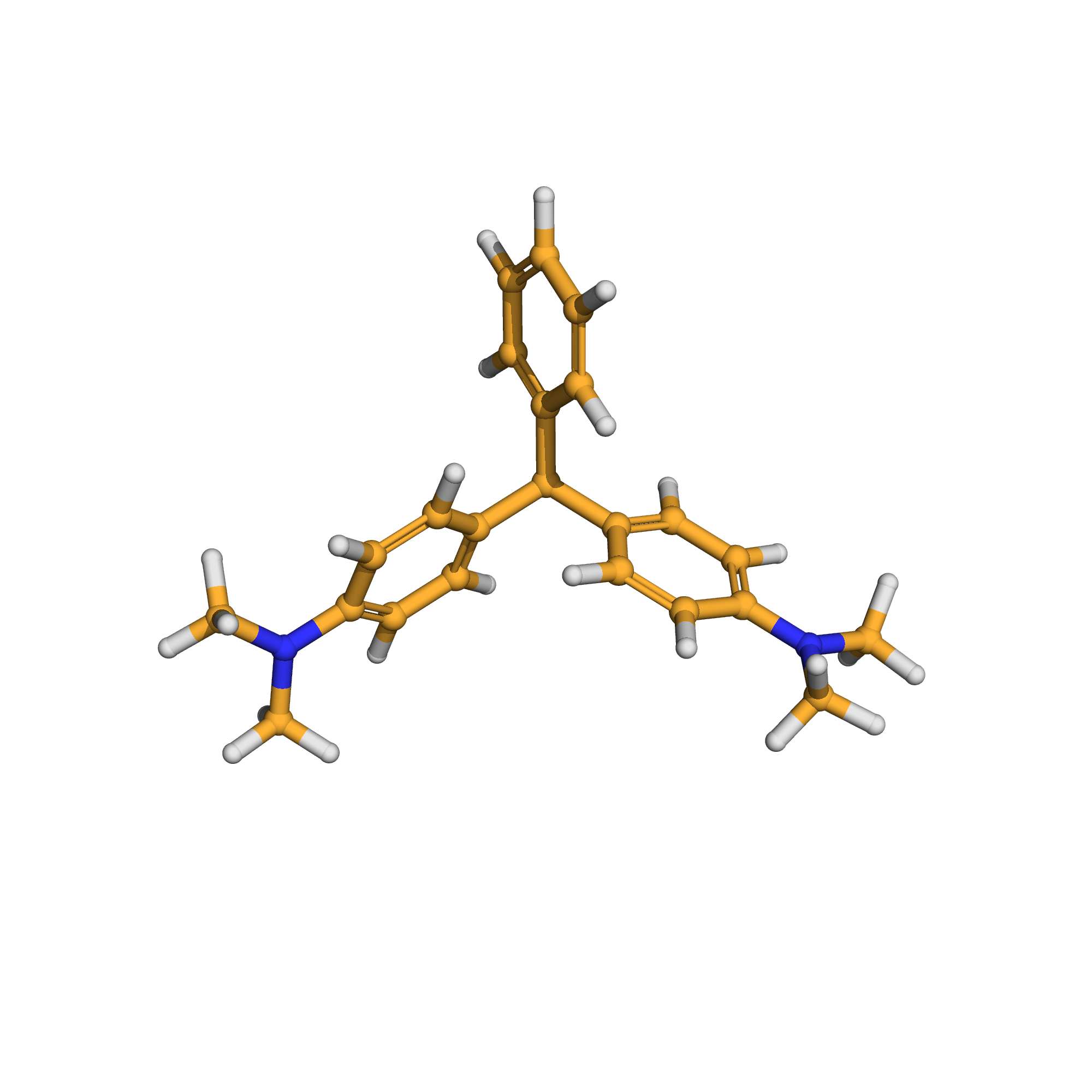
Canonical SMILES: CN(C)C1=CC=C(C=C1)C(C2=CC=CC=C2)C3=CC=C(C=C3)N(C)C
Structural Properties:
Molecular Formula: C23H26N2
Molecular Weight: 330.475
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 2
Number of atoms different from hydrogen: 25
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Culp, S.J., Blankenship, L.R., Kusewitt, D.F., Doerge, D.R., Mulligan, L.T. and Beland, F.A., 1999. Toxicity and metabolism of malachite green and leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chemico-biological interactions, 122(3), pp.153-170. DOI: https://doi.org/10.1016/S0009-2797(99)00119-2. URL: https://www.sciencedirect.com/science/article/pii/S0009279799001192.
Doerge, D.R., Chang, H.C., Divi, R.L. and Churchwell, M.I., 1998. Mechanism for inhibition of thyroid peroxidase by leucomalachite green. Chemical research in toxicology, 11(9), pp.1098-1104. DOI: 10.1021/tx970226o. URL: https://pubs.acs.org/doi/abs/10.1021/tx970226o.
External Links

2D-structure
3D-structure