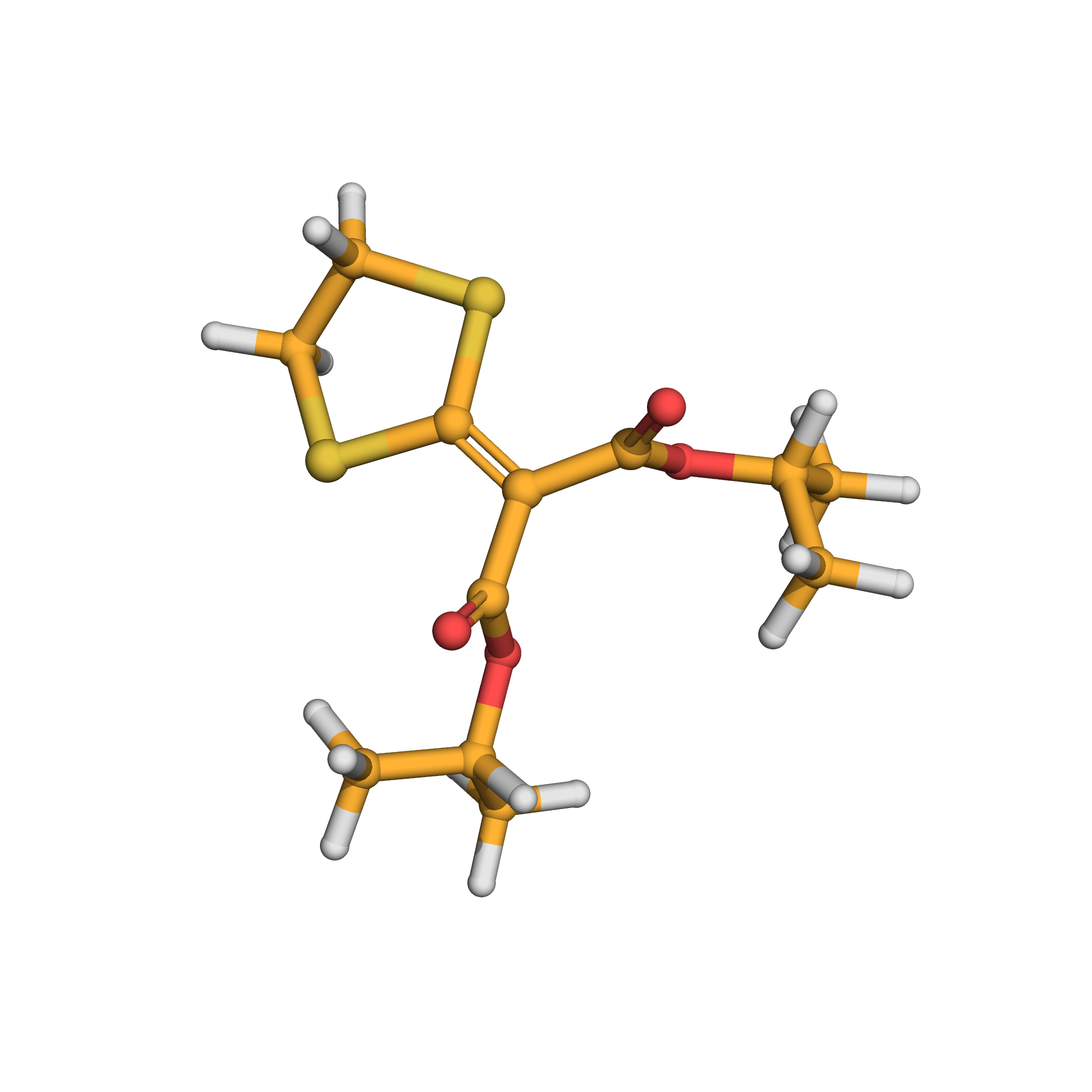
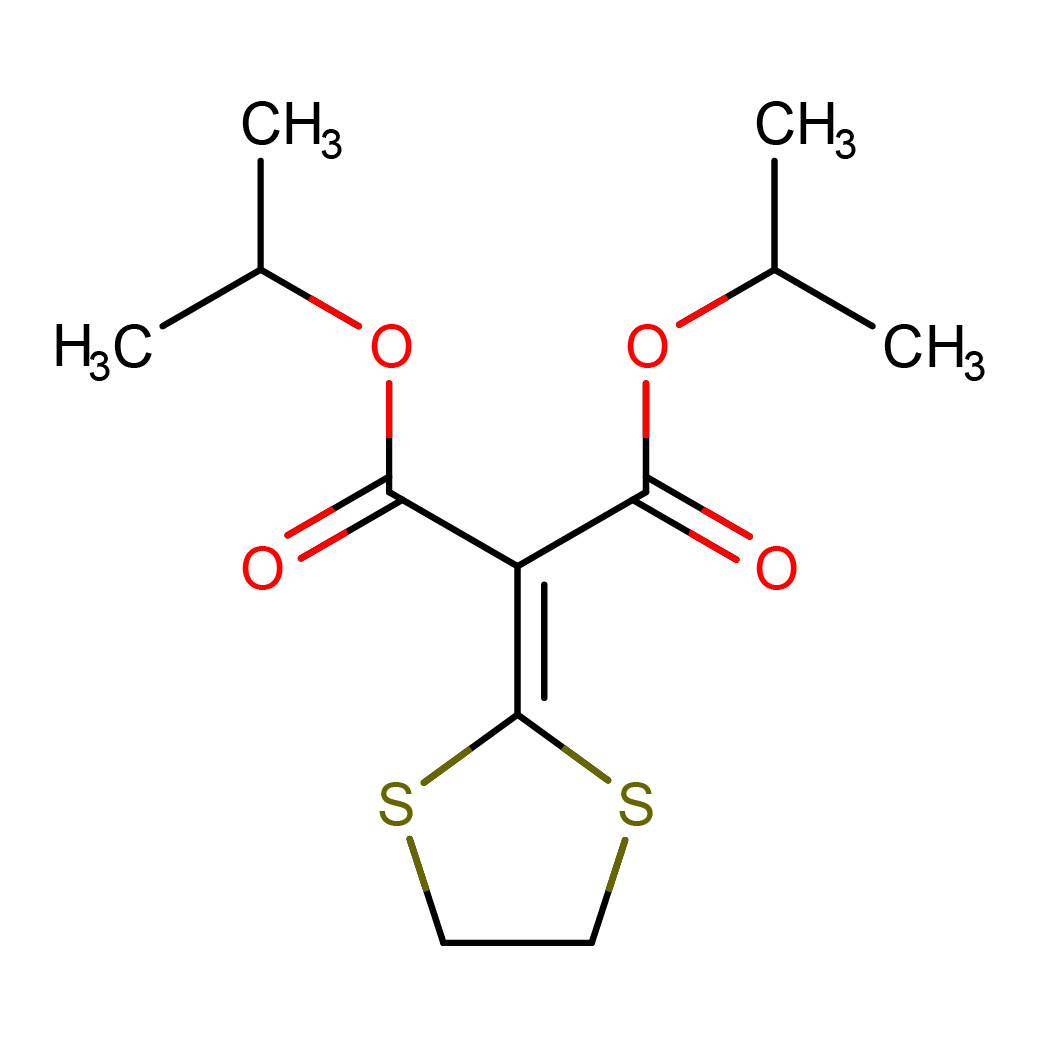
isoprothiolane
Synonyms: "diisopropyl 2-(1,3-dithiolan-2-ylidene)malonate", "di-isopropyl 1,3-dithiolane-2-ylidenemalonate", "bis(1-methylethyl) 1,3-dithiolan-2-ylidenepropanedioate", "diisopropyl 1,3-dithiolan-2-ylidenemalonate", "Fuji-one", "dipropan-2-yl 2-(1,3-dithiolan-2-ylidene)propanedioate", "IPT","Fudiolan", "Fujione", "IPT", "Fuji 1", "NKK 100", "NNF-109".
Source: Isoprothiolane is a systemic fungicide.
Identifiers:
IUPAC Name: dipropan-2-yl 2-(1,3-dithiolan-2-ylidene)propanedioate
CAS Number: 50512-35-1
PubChem ID: 39681
InChiKey: UFHLMYOGRXOCSL-UHFFFAOYSA-N
Canonical SMILES: CC(C)OC(=O)C(=C1SCCS1)C(=O)OC(C)C
Structural Properties:
Molecular Formula: C12H18O4S2
Molecular Weight: 290.392
Pharmacophore Features:
Number of bond donors: 0
Number of bond acceptors: 6
Number of atoms different from hydrogen: 18
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Hisato MI, 1998. Effects of the agrochemicals butachlor, pretilachlor and isoprothiolane on rat liver xenobioticmetabolizing enzymes. Xenobiotica 28(11):1029-1039. DOI: 10.1080/004982598238921. URL: http://www.tandfonline.com/doi/abs/10.1080/004982598238921.
Oh YJ, Jung YJ, Kang JW, Yoo YS. 2007. Investigation of the estrogenic activities of pesticides from Pal-dang reservoir by in vitro assay. Sci Total Environ 388(1-3):8-15. DOI: 10.1016/j.scitotenv.2007.07.013. URL: https://www.sciencedirect.com/science/article/pii/S0048969707007541?via%3Dihub.
External Links


2D-structure
3D-structure