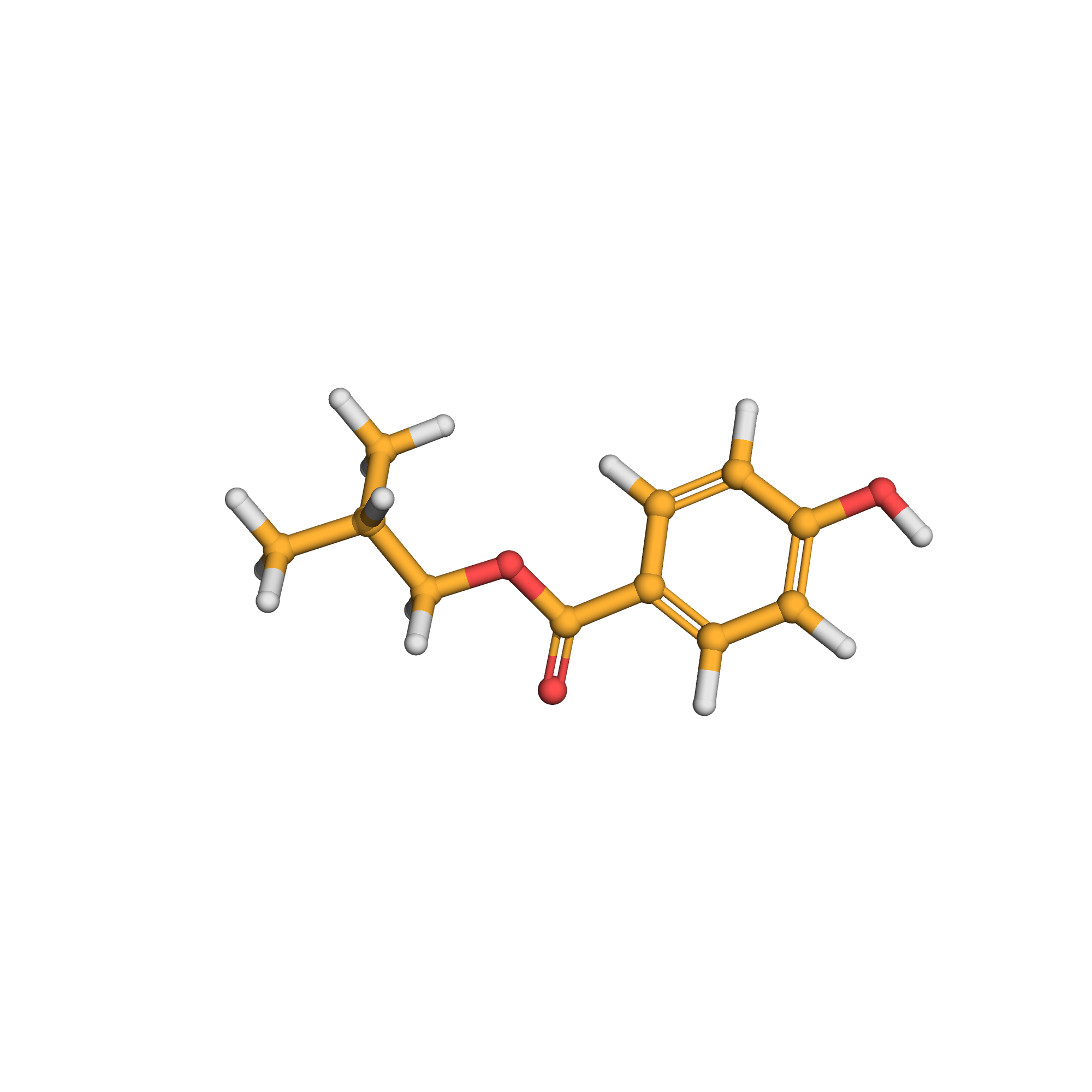
isobutyl 4-hydroxybenzoate
Synonyms: "isobutylparaben", "isobutyl p-hydroxybenzoate", "2-methylpropyl 4-hydroxybenzoate", "isobutyl paraben", "isobutyl-4-hydroxybenzoate", "isobutyl parahydroxybenzoate", "4-hydroxybenzoic Acid Isobutyl ester", "p-oxybenzoesaureisobutylester", "p-Hydroxybenzoic acid isobutyl ester".
Source: Isobutyl 4-hydroxybenzoate is used as an anti-microbial agent. It is also used as antiseptics for cosmetic field.
Identifiers:
IUPAC Name: 2-methylpropyl 4-hydroxybenzoate
CAS Number: 42-47-02-3
PubChem ID: 20240
InChiKey: XPJVKCRENWUEJH-UHFFFAOYSA-N
Canonical SMILES: CC(C)COC(=O)C1=CC=C(C=C1)O
Structural Properties:
Molecular Formula: C11H14O3
Molecular Weight: 194.230
Pharmacophore Features:
Number of bond donors: 1
Number of bond acceptors: 3
Number of atoms different from hydrogen: 14
Downloads
2D structure (.sdf)
3D structure (.sdf)
3D structure (.mol2)
3D structure (.pdb)
3D structure (.pdbqt)
Search Similar molecules
Evidence Supporting This Chemical as an Endocrine Disruptor
TEDX List of Potential Endocrine Disruptors

Darbre PD, Byford JR, Shaw LE, Horton RA, Pope GS, Sauer MJ. 2002. Oestrogenic activity of isobutylparaben in vitro and in vivo. J Appl Toxicol 22(4):219-226. DOI: 10.1002/jat.860. URL: http://onlinelibrary.wiley.com/doi/10.1002/jat.860/abstractOkubo T, Yokoyama Y, Kano K, Kano I. 2001. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ER[alpha] and PR. Food Chem Toxicol 39(12):1225-1232. DOI: 10.1016/S0278-6915(01)00073-4. URL: https://www.sciencedirect.com/science/article/pii/S0278691501000734?via%3Dihub.
Terasaki M, Kamata R, Shiraishi F, Makino M. 2009. Evaluation of estrogenic activity of parabens and their chlorinated derivatives by using the yeast two-hybrid assay and the enzyme-linked immunosorbent assay. Environ Toxicol Chem 28(1):204-208. DOI: 10.1897/08-225.1. URL: http://onlinelibrary.wiley.com/doi/10.1897/08-225.1/fullVo TT, Jeung EB. 2009. An evaluation of estrogenic activity of parabens using uterine calbindin-d9k gene in an immature rat model. Toxicol Sci 112(1):68-77. DOI: 10.1093/toxsci/kfp176. URL: https://academic.oup.com/toxsci/article/112/1/68/1636403.
External Links



2D-structure
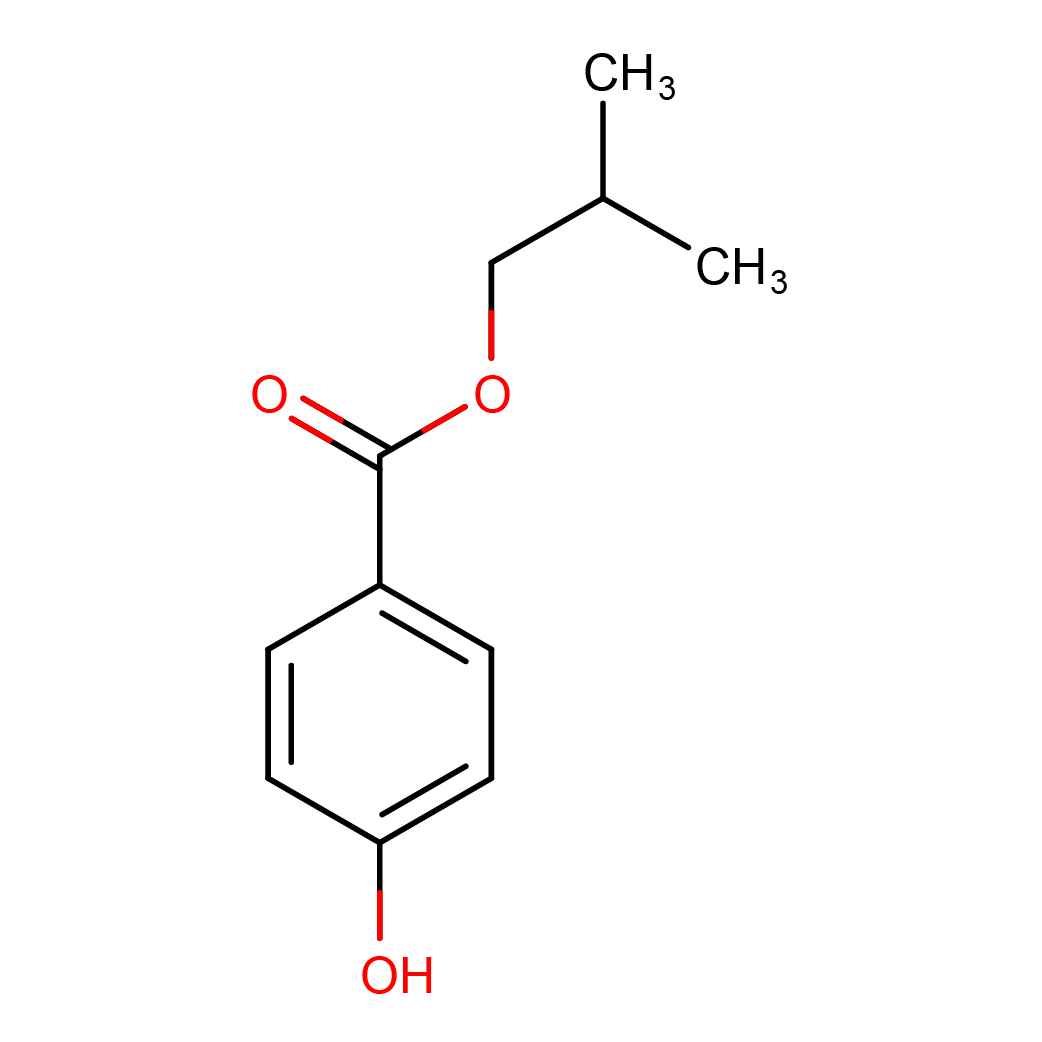
3D-structure